Exploring the Impact of Phenanthroline-Based Glycoconjugated Ru(II) Polypyridyl Photosensitizers on Metastasis-Related Processes
- PMID: 40433796
- PMCID: PMC12284616
- DOI: 10.1002/chem.202501105
Exploring the Impact of Phenanthroline-Based Glycoconjugated Ru(II) Polypyridyl Photosensitizers on Metastasis-Related Processes
Abstract
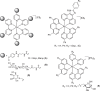
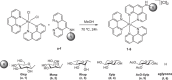
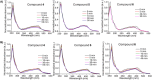
Novel water-soluble, phosphorescent ruthenium(II) polypyridyl-glycoconjugates of general formula [Ru{N-(1,10-phenanthroline)}2{N-(1,10-phenanthrolin-5-yl)-β-glycopyranosylamine}][Cl]2 (glycopyranosyl = D-glucopyranosyl (1), D-mannopyranosyl (2), L-rhamnopyranosyl (3), L-xylopyranosyl (4), and 2,3,4-tri-O-acetyl-L-xylopyranosyl (5)) and corresponding aglycone [Ru{N-(1,10-phenanthroline)}2{N-(1,10-phenanthrolin-5-amine)}][Cl]2 (6) have been synthesized and fully characterized. Their stability and behavior in physiological media have been assessed using ultraviolet visible spectroscopy (UV-Vis) and 1H nuclear magnetic resonance (¹H-NMR), revealing minor N-glycosidic cleavage and high photostability. The photocytotoxicity of the complexes has been evaluated in two different cancer (PC-3 and MCF-7) and one nontumorigenic (HFF-1) cell lines. While exhibiting no toxicity in the dark, some glycoconjugates achieve photoselectivity indexes of up to 40 upon blue-light irradiation. Remarkably, complexes 1-6 potently affect the metastatic phenotype of PC-3 cells, evidenced by the inhibition of migration in the wound healing assay and the increased resistance to trypsin detachment following photoactivation.
Keywords: antimetastatic; glycoconjugates; photodynamic therapy; photosensitizer; ruthenium(II) polypyridyl.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Clarke M. J., Bitler S., Rennert D., Buchbinder M., Kelman A. D., J. Inorg. Biochem. 1980, 12, 79. - PubMed
-
- Bratsos I., Gianferrara T., Alessio E., Hartinger C. G., Jakupec M. A., Keppler B. K., Bioinorg. Med. Chem. 2011, 151.
-
- Trondl R., Heffeter P., Kowol C. R., Jakupec M. A., Berger W., Keppler B. K., Chem. Sci. 2014, 5, 2925.
-
- Bergamo A., Sava G., Chem. Soc. Rev. 2015, 44, 8818. - PubMed
-
- Murray B. S., Babak M. V., Hartinger C. G., Dyson P. J., Coord. Chem. Rev. 2016, 306, 86.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

