A Human Engineered Heart Tissue-Derived Lipotoxic Diabetic Cardiomyopathy Model Revealed Early Benefits of Empagliflozin
- PMID: 40433797
- PMCID: PMC12376570
- DOI: 10.1002/advs.202503173
A Human Engineered Heart Tissue-Derived Lipotoxic Diabetic Cardiomyopathy Model Revealed Early Benefits of Empagliflozin
Abstract
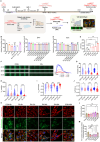
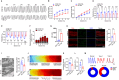
Diabetic cardiomyopathy (DbCM) is increasingly prevalent, but intervention targets remain unclear due to the lack of appropriate models and the complexity of risk factors. Here, this work establishes an in vitro assessment system for DbCM function using cardiomyocytes derived from human pluripotent stem cells and engineered heart tissue. This work finds high-fat status in complex diabetes risk factors majorly contributes most to cardiomyocyte death and contractile dysfunction. Notably, PA induced early electrophysiological abnormalities, and lately is associated with cardiac fibrosis, mitochondrial fission, and systolic and diastolic dysfunction at tissue level. Using this in vitro assessment system, this work finds that empagliflozin (EMPA), a first-line glucose-lowering drug, effectively alleviated early PA-induced cardiomyocyte injury. Treatment with EMPA enhanced abnormal diastolic and electrophysiological functions in the PA-hEHT model and significantly reduced endoplasmic reticulum stress, and apoptosis. Furthermore, these promising results are confirmed in a type 2 diabetes mellitus mouse model, reinforcing the potential of EMPA as a therapeutic option to alleviate cardiomyocyte injury under diabetic conditions. These findings suggest that this work has developed an engineered model of diabetic cardiomyopathy that mimics the various stages of lipotoxic myocardial injury and support the use of EMPA as a potential therapeutic option for diabetic or lipotoxic cardiomyopathy.
Keywords: diabetic cardiomyopathy; diastolic dysfunction; empagliflozin; hEHT; in vitro model.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Magliano D. J., Boyko E. J., IDF Diabetes Atlas, in Idf Diabetes Atlas, International Diabetes Federation © International Diabetes Federation, Brussels: 2021.
-
- Wong N. D., Sattar N., Nat. Rev. Cardiol. 2023, 20, 685. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
