Key Connectomes and Synaptic-Compartment-Specific Risk Genes Drive Pathological α-Synuclein Spreading
- PMID: 40433888
- PMCID: PMC12224943
- DOI: 10.1002/advs.202413052
Key Connectomes and Synaptic-Compartment-Specific Risk Genes Drive Pathological α-Synuclein Spreading
Abstract
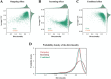
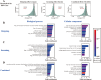
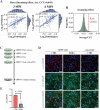
Previous studies have suggested that pathological α-synuclein (α-Syn) mainly transmits along the neuronal network, but several key questions remain unanswered: 1) How many and which connections in the connectome are necessary for predicting the progression of pathological α-Syn? 2) How to identify risk genes that affect pathology spreading functioning at presynaptic or postsynaptic regions, and are these genes enriched in different cell types? Here, these questions are addressed with novel mathematical models. Strikingly, the spreading of pathological α-Syn is predominantly determined by the key subnetworks composed of only 2% of the strongest connections in the connectome. Genes associated with the selective vulnerability of brain regions to pathological α-Syn transmission are further analyzed to distinguish those functioning at presynaptic versus postsynaptic regions. Those risk genes are significantly enriched in microglial cells of presynaptic regions and neurons of postsynaptic regions. Gene regulatory network analyses are then conducted to identify "key drivers" of genes responsible for selective vulnerability and overlapping with Parkinson's disease risk genes. By identifying and discriminating between key gene mediators of transmission operating at presynaptic and postsynaptic regions, this study has demonstrated for the first time that these are functionally distinct processes.
Keywords: key connectomes; mathematical models; pathological α‐synuclein; risk genes; systems biology analyses.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Update of
-
Distinguish risk genes functioning at presynaptic or postsynaptic regions and key connectomes associated with pathological α-synuclein spreading.bioRxiv [Preprint]. 2025 Mar 13:2025.03.11.642462. doi: 10.1101/2025.03.11.642462. bioRxiv. 2025. Update in: Adv Sci (Weinh). 2025 Jul;12(25):e2413052. doi: 10.1002/advs.202413052. PMID: 40161679 Free PMC article. Updated. Preprint.
References
-
- El‐Agnaf O. M., Salem S. A., Paleologou K. E., Curran M. D., Gibson M. J., Court J. A., Schlossmacher M. G., Allsop D., FASEB J. 2006, 20, 419. - PubMed
-
- Mollenhauer B., Cullen V., Kahn I., Krastins B., Outeiro T. F., Pepivani I., Ng J., Schulz‐Schaeffer W., Kretzschmar H. A., McLean P. J., Exp. Neurol. 2008, 213, 315. - PubMed
MeSH terms
Substances
Grants and funding
- R01-NS111378/NIH/NINDS
- MJFF-009049/Michael J. Fox Foundation
- Noble Family Innovation Fund
- RF1AG072753/NIH/NIA
- RF1 AG062196/AG/NIA NIH HHS/United States
- R01-NS128964/GF/NIH HHS/United States
- R01 AG072753/AG/NIA NIH HHS/United States
- R21 AG087921/AG/NIA NIH HHS/United States
- R01 NS111378/NS/NINDS NIH HHS/United States
- R01 NS117148/NS/NINDS NIH HHS/United States
- UL1TR001881/NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI
- R01-NS117148/NIH/NINDS
- Elman Family Fund
- RF1AG062196/NIH/NIA
- MJFF-023428/Michael J. Fox Foundation
- MJFF-024074/Michael J. Fox Foundation
- R21AG087921/GF/NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 NS128964/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
