Neutrophil-Mimicking Nanomedicine Eliminates Tumor Intracellular Bacteria and Enhances Chemotherapy on Liver Metastasis of Colorectal Cancer
- PMID: 40433907
- PMCID: PMC12376532
- DOI: 10.1002/advs.202504188
Neutrophil-Mimicking Nanomedicine Eliminates Tumor Intracellular Bacteria and Enhances Chemotherapy on Liver Metastasis of Colorectal Cancer
Abstract
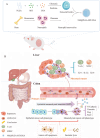
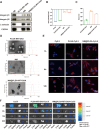
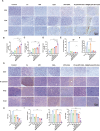
Fusobacterium nucleatum (Fn) enrichment has been identified in colorectal cancer and its liver metastases. In this study, we found that Fn predominantly accumulated within colorectal cancer cells, correlating with colorectal cancer liver metastasis. Clinically, the administration of high doses of antibiotics and chemotherapeutic agents can disrupt the balance of the host microbiota. To address this clinical challenge, metronidazole (MTI) and oxaliplatin (OXA) are encapsulated within poly (lactic-co-glycolic acid) (PLGA) nanoparticles. Neutrophil membrane vesicles are extracted from murine bone marrow and coated with these nanoparticles (NM@PLGA-MTI-OXA), creating neutrophil-mimetic nanoparticles with dual targeting capabilities for antibacterial and anticancer purposes. The neutrophil membrane coating, compared with free drugs, is found to enhance nanoparticle uptake by tumor cells, facilitating intracellular bacterial elimination and tumor cell death. Further experiments reveal that NM@PLGA-MTI-OXA reverses the Fn-induced epithelial-mesenchymal transition (EMT) in tumor cells during metastasis and remodels the immunosuppressive microenvironment, suppressing colorectal cancer and liver metastasis development while minimizing broad-spectrum damage to the commensal microbiota.
Keywords: colorectal cancer; liver metastasis; nanomedicine; tumor microbiome.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., Jemal A., CA: Cancer J. Clin. 2024, 74, 229. - PubMed
-
- Murphy C. C., Zaki T. A., Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 25. - PubMed
-
- Morris V. K., Kennedy E. B., Baxter N. N., Benson A. B., Cercek A., Cho M., Ciombor K. K., Cremolini C., Davis A., Deming D. A., Fakih M. G., Gholami S., Hong T. S., Jaiyesimi I., Klute K., Lieu C., Sanoff H., Strickler J. H., White S., Willis J. A., Eng C., J. Clin. Oncol. 2023, 41, 678. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
