Structural basis of G-quadruplex recognition by a camelid antibody fragment
- PMID: 40433978
- PMCID: PMC12117401
- DOI: 10.1093/nar/gkaf453
Structural basis of G-quadruplex recognition by a camelid antibody fragment
Abstract
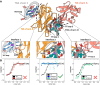
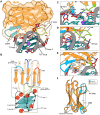
Apart from the iconic Watson-Crick duplex, DNA can fold into different noncanonical structures, of which the most studied are G-quadruplexes (G4s). Despite mounting structural and biophysical evidence, their existence in cells was controversial until their detection using G4-specific antibodies. However, it remains unknown how antibodies recognize G4s at the molecular level and why G4-specific antibodies have low selectivity and are unable to distinguish different G4 sequences. Here, we present the crystal structure of a nanobody bound to the archetypical G4 structure, the thrombin-binding aptamer (TBA). The nanobody exhibits strong selectivity against different G4 sequences and utilizes an unusual scaffold-based paratope, with very limited involvement of complementarity-determining region. The nanobody effectively mimics the binding interface of thrombin, a natural binding partner of TBA, by using isosteric interactions at key positions. The presented structure sheds light on the molecular basis of how antibodies, essential G4-detection tools, recognize noncanonical G4 structures.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures




References
-
- Largy E, Mergny J-L, Gabelica V. Sigel A, Sigel H, Sigel R Role of alkali metal ions in G-quadruplex nucleic acid structure and stability. Metal Ions in Life Sciences. 2016; 16:Cham: Springer; 203–58. - PubMed
-
- Winnerdy FR, Phan AT. Neidle S Quadruplex structure and diversity. Annual Reports in Medicinal Chemistry. 2020; 54:Cambridge, US: Academic Press; 45–73.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

