Unveiling Formation Pathways of Ternary I-III-VI CuInS2 Quantum Dots and Their Effect on Photoelectrochemical Hydrogen Generation
- PMID: 40434047
- PMCID: PMC12376712
- DOI: 10.1002/advs.202500829
Unveiling Formation Pathways of Ternary I-III-VI CuInS2 Quantum Dots and Their Effect on Photoelectrochemical Hydrogen Generation
Abstract
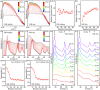
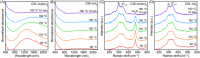
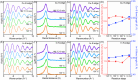
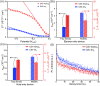
Understanding the formation mechanisms of semiconductor nanocrystal quantum dots (QDs) is essential for fine-tuning their optical and electrical properties. Despite their potential in solar energy conversion, the synthesis processes and resulting properties of ternary I-III-VI QDs remain underexplored due to the complex interplay among their constituent elements. Herein, the formation mechanism of ternary I-III-VI CuInS2 QDs is investigated, and a direct correlation between their synthesis pathways and photoelectrochemical hydrogen generation performance is established. Two distinct formation pathways governed by the Lewis acid strength of the precursors are revealed. Precursors with weaker Lewis acid strength, such as indium acetate-alkylamine complexes, induce the nucleation of Cu x S phases, which subsequently transform into CuInS2 QDs. Conversely, exemplified by indium iodide-alkylamine complexes, precursors with stronger Lewis acid strength enable the simultaneous incorporation of all elements during nucleation, resulting in the direct formation of CuInS2 QDs. Notably, QDs synthesized through this direct pathway exhibit significantly improved electrical properties with lower electron trap densities, resulting in outstanding photoelectrochemical hydrogen production with an excellent photocurrent density of 11.3 mA cm-2 at 0.6 VRHE when used as sensitizers in photoanodes. These findings highlight the critical role of formation pathways in tailoring the properties of ternary I-III-VI QDs.
Keywords: I–III–VI; formation mechanism; hydrogen production; photoelectrochemical properties; quantum dots.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Murray C. B., Norris D. J., Bawendi M. G., J. Am. Chem. Soc. 1993, 115, 8706.
-
- Gu F., Guo W., Yuan Y., Deng Y.‐P., Jin H., Wang J., Chen Z., Pan S., Chen Y., Wang S., Adv. Mater. 2024, 36, 2313096. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
