The Impact of Conjugation Mode and Site on Tubulysin Antibody-Drug-Conjugate Efficacy and Stability
- PMID: 40434212
- PMCID: PMC12368874
- DOI: 10.1002/open.202400522
The Impact of Conjugation Mode and Site on Tubulysin Antibody-Drug-Conjugate Efficacy and Stability
Abstract
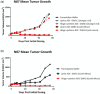
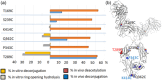
Antibody-drug conjugates (ADCs) represent a prominent class of biotherapeutics engineered to selectively deliver cytotoxic payloads to tumors, thereby facilitating targeted cell killing. While first-generation ADCs, created by conjugating payloads to surface-accessible lysine or hinge-cysteine residues, have achieved clinical success, several site-specific ADCs with defined drug-to-antibody ratios are currently under clinical investigation. Herein, the efficacy, stability, and pharmacokinetics of ADCs generated by attaching the drug linker to surface-exposed lysine residues, hinge-cysteine residues, and the C'E loop in the CH2 domain (mediated by bacterial transglutaminase) using a tubulysin payload are compared. In N87 xenograft mice, the order of efficacy is C'E loop > hinge-cysteine > lysine-conjugated ADCs. Among the three ADCs evaluated, the site-specific ADC demonstrates superior in vivo stability (minimal payload-linker deconjugation and limited payload metabolism/deacetylation) and favorable pharmacokinetics (longer half-life, low clearance, high exposure). In contrast, the lysine-conjugated ADC exhibits the least stability and poorest pharmacokinetics, which directly correlate with its efficacy. Further investigation into cysteine-engineered site-specific ADCs with payloads conjugated at various sites confirms that both the conjugation chemistry and the site of conjugation significantly influence the in vivo stability and pharmacokinetics of site-specific ADCs.
Keywords: antibody‐drug conjugates; deconjugation; payload metabolism; site‐specific conjugations; tubulysin.
© 2025 The Author(s). ChemistryOpen published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khongorzul P., Ling C. J., Khan F. U., Ihsan A. U., Zhang J., Mol. Cancer Res. 2020, 18, 3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

