Chalcogenide Materials in Water Purification: Advances in Adsorptive and Photocatalytic Removal of Organic Pollutants
- PMID: 40434237
- PMCID: PMC12288794
- DOI: 10.1002/smll.202501378
Chalcogenide Materials in Water Purification: Advances in Adsorptive and Photocatalytic Removal of Organic Pollutants
Abstract
Chalcogenide-based materials, known for their unique physicochemical properties, emerge as promising solutions for the removal of hazardous organic pollutants, such as dyes, pharmaceuticals, pesticides, and herbicides, from water and wastewater. This review examines the latest developments in the synthesis, structural optimization, and application of chalcogenide materials for environmental remediation. The past decade has witnessed remarkable advances in controlling the composition and structure of chalcogenide materials at the atomic level. The development of precise synthetic methods enables the creation of complex hierarchical structures, heterojunctions, and hybrid materials, leading to significant improvements in photocatalytic efficiency, stability, and selectivity for various environmental applications. Key emphasis is placed on adsorption and photocatalysis as green technologies, offering efficient pathways for pollutant removal. Mechanistic insights into the interactions between chalcogenide materials and contaminants are explored, providing a comprehensive understanding of their performance. Furthermore, challenges such as toxicity, scalability, and operational stability are discussed alongside future prospects for integrating these materials into industrial-scale water treatment systems. This review aims to inspire continued innovation in sustainable water purification technologies using chalcogenides.
Keywords: adsorption; metal chalcogenides; organic pollutants; photocatalytic; water/wastewater treatment.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













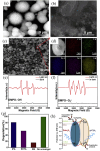
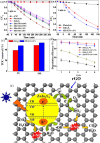

Similar articles
-
Biochar-based catalysts: an efficient and sustainable approach for water remediation from organic pollutants via advanced oxidation processes.J Environ Manage. 2025 Aug;390:126245. doi: 10.1016/j.jenvman.2025.126245. Epub 2025 Jun 21. J Environ Manage. 2025. PMID: 40544812 Review.
-
Functionalization of halloysite nanotubes as remediation agents for organic and inorganic contaminants: Insights into synthesis routes, removal techniques, and eco-friendly perspectives.Sci Total Environ. 2025 Aug 10;989:179771. doi: 10.1016/j.scitotenv.2025.179771. Epub 2025 Jun 11. Sci Total Environ. 2025. PMID: 40505512 Review.
-
Green and sustainable metal-organic frameworks (MOFs) in wastewater treatment: A review.Environ Res. 2025 Oct 1;282:122087. doi: 10.1016/j.envres.2025.122087. Epub 2025 Jun 4. Environ Res. 2025. PMID: 40480361 Review.
-
[Recent applications of porous-material-based adsorbents for extracting pesticide residues from environmental and foodstuff samples].Se Pu. 2025 Jul;43(7):713-725. doi: 10.3724/SP.J.1123.2024.12009. Se Pu. 2025. PMID: 40610766 Free PMC article. Review. Chinese.
-
Recent progress and function of nanocellulose in enhancing semiconductor-based photocatalytic wastewater treatment.Int J Biol Macromol. 2025 Aug;319(Pt 3):145565. doi: 10.1016/j.ijbiomac.2025.145565. Epub 2025 Jun 25. Int J Biol Macromol. 2025. PMID: 40578634 Review.
References
-
- Bamisaye A., Abati S. M., Ige A. R., Etafo N. O., Alli Y. A., Bamidele M. O., Okon‐Akan O. A., Adegoke K. A., Abiola‐Kuforiji O. T., Idowu M. A., Bello O. S., Chemosphere 2024, 367, 143569. - PubMed
-
- Gold V. F., Bamisaye A., Adesina M. O., Adegoke K. A., Ige A. R., Adeleke O., Bamidele M. O., Alli Y. A., Oyebamiji A. K., Ogunlaja O. O., J. Dispersion Sci. Technol., 1, 1.
-
- Sarma G. V. S. S., Chavali M., Nikolova M. P., Enamala M. K., Kuppan C., in Micro and Nano Technologies (Ed: M.M.B.T.‐C.‐B.N. as Khan P.), Elsevier, Amsterdam, Netherlands, 2021, pp. 77–103.
-
- Mondal S., Aikat K., Halder G., Ecol. Eng. 2016, 92, 158.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

