Spatiotemporal calcium signaling patterns underlying opposing effects of histamine and TAS2R agonists in airway smooth muscle
- PMID: 40434402
- PMCID: PMC12188627
- DOI: 10.1152/ajplung.00058.2025
Spatiotemporal calcium signaling patterns underlying opposing effects of histamine and TAS2R agonists in airway smooth muscle
Abstract
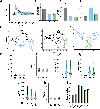
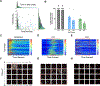
Intracellular calcium (Ca2+) release via phospholipase C (PLC) following G-protein-coupled receptor (GPCR) activation is typically linked to membrane depolarization and airway smooth muscle (ASM) contraction. However, recent findings show that bitter taste receptor agonists, such as chloroquine (CQ), induce a paradoxical and potent relaxation response despite activating the Ca2+ signaling pathway. This relaxation has been hypothesized to be driven by a distinct compartmentalization of calcium ions toward the cellular periphery, subsequently leading to membrane hyperpolarization, in contrast to the contractile effects of histamine. In this study, we further investigate the spatiotemporal dynamics of Ca2+ signaling in ASM cells using single-cell microscopy and deep learning-based segmentation, integrating the results into a comprehensive model of ASM ion channel dynamics to compare the effects of histamine, CQ, and flufenamic acid (FFA). Our results show that histamine induces a strong, synchronized calcium release, nearly twice as high as that of CQ, which produces a sustained but lower-magnitude response. Per-cell analysis reveals more variable and asynchronous Ca2+ signaling for CQ and FFA, with higher entropy compared with histamine. Integrating these findings into an ASM ion channel model, we observed that histamine-mediated Ca2+ release activates voltage-gated Ca2+ and Na+ channels (leading to depolarization). In contrast, CQ preferentially engages BKCa, SKCa, and chloride channels (promoting hyperpolarization). These findings provide insights into the unique mechanisms by which bitter taste receptor agonists can modulate ASM tone, offering potential therapeutic strategies for relaxing ASM and alleviating airway hyperresponsiveness in conditions such as asthma.NEW & NOTEWORTHY Using machine-learning methods, these studies identify spatiotemporal differences in calcium responses between agonists of Gq-coupled receptors and bitter taste receptors in airway smooth muscle cells. The findings provide deeper insights into the mechanism of action of bitter tastant-induced airway smooth muscle relaxation.
Keywords: TAS2R; airway smooth muscle; calcium; chloroquine.
Conflict of interest statement
Figures


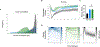

Similar articles
-
Molecular mechanism of bitter taste receptor agonist-mediated relaxation of airway smooth muscle.FASEB J. 2024 Jul 31;38(14):e23842. doi: 10.1096/fj.202400452R. FASEB J. 2024. PMID: 39037554 Free PMC article.
-
Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2016 Feb 15;310(4):L365-76. doi: 10.1152/ajplung.00373.2015. Epub 2015 Dec 18. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26684251 Free PMC article.
-
Role of STIM1 in stretch-induced signaling in human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2024 Aug 1;327(2):L150-L159. doi: 10.1152/ajplung.00370.2023. Epub 2024 May 21. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38771147 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Deshpande DA, and Penn RB (2006) Targeting G protein-coupled receptor signaling in asthma. Cell Signal 18, 2105–2120 - PubMed
-
- Shieh CC, Petrini MF, Dwyer TM, and Farley JM (1991) Concentration-dependence of acetylcholine-induced changes in calcium and tension in swine trachealis. J Pharmacol Exp Ther 256, 141–148 - PubMed
-
- Sims SM, Jiao Y, and Zheng ZG (1996) Intracellular calcium stores in isolated tracheal smooth muscle cells. Am J Physiol 271, L300–309 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

