Perceptions and Attitudes Toward Oral Semaglutide Among Japanese Physicians and Individuals with Type 2 Diabetes: A Web-Based Survey
- PMID: 40434552
- PMCID: PMC12182544
- DOI: 10.1007/s13300-025-01739-2
Perceptions and Attitudes Toward Oral Semaglutide Among Japanese Physicians and Individuals with Type 2 Diabetes: A Web-Based Survey
Abstract
Introduction: The oral formulation of the glucagon-like peptide-1 receptor agonist semaglutide has dosing requirements that could impact adherence. We compared perceptions of physicians and individuals with type 2 diabetes (T2D) in Japan, before and after initiating oral semaglutide, with respect to adherence to dosing requirements.
Methods: In this observational study, online questionnaires were completed by treating physicians and adults with T2D who had received either oral semaglutide or other oral antidiabetic medications for ≥ 6 months. Physicians reported the expected adherence of their patients to oral semaglutide and expected patient difficulties around the dosing requirements prior to (baseline) and after (time of survey) initiating oral semaglutide. Patient-reported adherence and difficulties experienced with the dosing requirements were assessed after oral semaglutide was initiated.
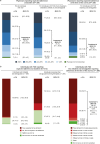
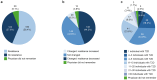
Results: Overall, 330 physicians and 412 individuals with T2D responded. There was a statistically significant difference (P < 0.001) between baseline and time of survey in the distribution of physicians who expected that ≥ 80% or < 80% of their patients would adhere to oral semaglutide, with 17.2% of physicians expecting ≥ 80% of their patients to be adherent before treatment initiation versus 44.6% reporting ≥ 80% of their patients to be adherent after treatment initiation. There was also a statistically significant difference (P < 0.001) in the distribution of expected versus reported adherence among individuals who received oral semaglutide. After semaglutide initiation, 95.2% of patients reported missing ≤ 1 dose/week. Before prescribing oral semaglutide, 186 (56.4%) physicians were resistant to it, due to the dosing requirements. After prescribing oral semaglutide, 146 (44.2%) reported their resistance had decreased, whereas only 28 (8.5%) reported increased resistance.
Conclusions: We identified an improvement in physician perceptions of oral semaglutide adherence due to dosing requirements following semaglutide initiation. Our findings suggest that individuals with T2D in Japan are capable of adhering to the dosing requirements of oral semaglutide.
Keywords: Drug administration; Japan; Medication adherence; Oral semaglutide; Patient attitude; Physician perspective.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Ryo Suzuki: received lecture fees from Astellas Pharma Inc., Eli Lilly Japan K.K., Kowa Company Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Novo Nordisk Pharma Ltd., Sanofi K.K., Sumitomo Pharma Co., Ltd., Teijin Healthcare Ltd.; and grants from Nippon Boehringer Ingelheim Co., Ltd., Krishant Chand and Yuu Taguchi are employed by Novo Nordisk Pharma Ltd. Yuu Taguchi is a shareholder in Novo Nordisk Pharma Ltd. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices. The study protocol, consent forms, and questionnaires were approved by an independent ethics committee of the Research Institute of Healthcare Data Science (approval number RI2023005) on December 4, 2023. All respondents provided informed consent online, and were made aware and gave consent that their responses would be published in a medical journal. All personal information was managed by Macromill, Inc. (Tokyo, Japan), was used solely for the purposes of this study, and was not provided to the study sponsor.
Figures


References
-
- Pharmaceuticals and Medical Devices Agency. Rybelsus® tablets package insert (4th edition) (Japanese). 2024. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/620023_24.... Accessed December 1, 2024.
-
- Pharmaceuticals and Medical Devices Agency. Ozempic® subcutaneous injection package insert (5th edition) (Japanese). 2024. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/620023_24.... Accessed December 1, 2024.
LinkOut - more resources
Full Text Sources

