Atrial helix-fixation leadless pacemaker: real-world single-chamber implant experience
- PMID: 40434578
- PMCID: PMC12399728
- DOI: 10.1007/s10840-025-02041-8
Atrial helix-fixation leadless pacemaker: real-world single-chamber implant experience
Abstract
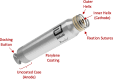
Background: Single -chamber ventricular leadless pacemakers (LPs) are well established. A dual-chamber LP system has recently become available with distinct atrial and ventricular devices. Single-chamber atrial pacing with transvenous devices is infrequent due to future upgrade concerns. This multi-center study evaluated the initial real-world use of the atrial LP by itself to treat isolated sinus node dysfunction (SND).
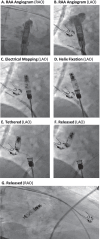
Methods: SND patients with normal PR interval and AV conduction to be implanted with atrial LPs after commercial US release were consecutively included. Procedural characteristics were evaluated, and electrical parameters were measured during pre-fixation mapping, post-fixation tether mode, after LP release, and before patient discharge. Acute, 30-day procedure- or device-related complications were noted.
Results: Aveir AR devices were implanted per standard local practice (N = 75 patients; 3 centers; 72 ± 13 years; 52% male; 92% de novo) with 100% success and placed predominantly in the right atrial appendage base (83%). The total procedure duration (from first incision to final suture) was 36 ± 33 min and the cumulative fluoroscopy duration was 7 ± 8 min. Pre-fixation mapping made repositioning unnecessary in 95% of implants. Pacing capture threshold at 0.4 ms pulse width, sensed amplitude, and impedance values of 0.6 ± 0.6 V, 2.9 ± 1.5 mV, and 329 ± 46 Ω, respectively, were measured prior to patient discharge. Capture threshold and sensed amplitude had improved significantly from LP release to patient discharge. No acute complications were observed.
Conclusion: This initial real-world experience implanting the helix-fixation, single-chamber, atrial LP in SND patients demonstrated safe and efficient implantation, clinically acceptable electrical metrics, and no acute complications.
Keywords: Atrial pacemaker; Aveir; Helix fixation; Implant procedure; Leadless pacemaker; Pacemaker complications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: DGN has served on the advisory board, served as a consultant, or received honoraria, research grants, or support from Medtronic Inc., Boston Scientific Corporation, Abbott, Biosense Webster, Siemens and Volta. NB, LG, and KR are employees of Abbott. CH served as a consultant or received honoraria, research grants, or support from Abbott and Biotronik.
Figures

References
-
- Tjong FVY, Reddy VY. Permanent leadless cardiac pacemaker therapy: a comprehensive review. Circulation. 2017;135(15):1458–70. 10.1161/CIRCULATIONAHA.116.025037. - PubMed
-
- Knops RE, et al. A dual-chamber leadless pacemaker. N Engl J Med. 2023. 10.1056/NEJMoa2300080. - PubMed
-
- Hindricks G, et al. Six-month electrical performance of the first dual-chamber leadless pacemaker. Heart Rhythm. 2024. 10.1016/j.hrthm.2024.04.091. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

