High-altitude hypoxia exacerbates chemotherapy-induced myelosuppression by lowering serum G-CSF/GM-CSF and regulating apoptosis and proliferation
- PMID: 40434597
- PMCID: PMC12119416
- DOI: 10.1007/s12672-025-02611-2
High-altitude hypoxia exacerbates chemotherapy-induced myelosuppression by lowering serum G-CSF/GM-CSF and regulating apoptosis and proliferation
Abstract
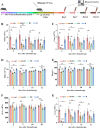
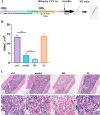
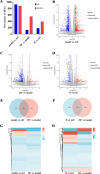
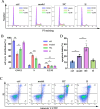
The unique hypoxic environment in high-altitude regions is increasingly drawing attention for its impact on the health of residents, particularly in patients post-chemotherapy. This study aimed to investigate the effects and potential mechanisms of high-altitude hypoxia on myelosuppression following chemotherapy, with the goal of providing a theoretical basis for clinical treatment. A retrospective clinical study of 80 patients with breast cancer revealed that patients in the plateau exhibited a significantly higher incidence of grade 3 or higher neutropenia and any level of neutropenia post-chemotherapy than those in the plain, with propensity score matching (PSM) confirming these associations. Animal experiments revealed that high-altitude hypoxia reduced the white blood cell (WBC) count, granulocyte count, lymphocyte count, and number of bone marrow nucleated cells (BMNCs) in cyclophosphamide (CTX)-treated mice. Additionally, high-altitude hypoxia induced a significant reduction in the proliferation index and an elevation in apoptosis rates in BMNCs. High-altitude hypoxia also significantly reduced serum levels of granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Transcriptomic analysis of BMNCs demonstrated that high-altitude hypoxia might modulate the hematopoietic function in CTX-induced myelosuppression mice through pathways related to hematopoiesis, such as porphyrin metabolism, hematopoietic cell lineage, ECM-receptor interaction, and PI3K-Akt signaling pathway. Our results suggest that high-altitude hypoxia exacerbates chemotherapy-induced myelosuppression, possibly through reducing the serum level of G-CSF/GM-CSF and regulating apoptosis and proliferation by PI3K-Akt signaling pathway, highlighting that cancer patients undergoing chemotherapy in hypoxic environments may require enhanced supportive care to mitigate these adverse effects.
Keywords: Chemotherapy; Hematopoietic function; High-altitude hypoxia; Myelosuppression.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Affiliated Hospital of Qinghai University (approval number P-SL-2023-447). Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent for publication: All authors agreed to publish. Competing interest: The authors declare no competing interests.
Figures

Similar articles
-
Granulocyte-macrophage colony-stimulating factor (GM-CSF) priming of high-dose etoposide and cyclophosphamide: a pilot trial.Exp Hematol. 1996 Oct;24(12):1363-8. Exp Hematol. 1996. PMID: 8913281
-
Comparative effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) on priming peripheral blood progenitor cells for use with autologous bone marrow after high-dose chemotherapy.Blood. 1993 Apr 1;81(7):1709-19. Blood. 1993. PMID: 7681699
-
Hematopoietic recovery following high-dose combined alkylating-agent chemotherapy and autologous bone marrow support in patients in phase-I clinical trials of colony-stimulating factors: G-CSF, GM-CSF, IL-1, IL-2, M-CSF.Ann Hematol. 1993 Dec;67(6):267-76. doi: 10.1007/BF01696346. Ann Hematol. 1993. PMID: 7506580 Clinical Trial.
-
Hematologic Growth Factors.2016 Aug 16. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2016 Aug 16. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31644052 Free Books & Documents. Review.
-
G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors.FEBS Open Bio. 2022 Jul;12(7):1268-1285. doi: 10.1002/2211-5463.13445. Epub 2022 Jun 9. FEBS Open Bio. 2022. PMID: 35612789 Free PMC article. Review.
References
-
- Gatterer H, Villafuerte FC, Ulrich S, Bhandari SS, Keyes LE, Burtscher M. Altitude illnesses. Nat Rev Dis Primers. 2024;10(1):43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
