Network Meta-Analysis of Pharmacological Therapies for Long-Term Prophylactic Treatment of Patients with Hereditary Angioedema
- PMID: 40434599
- PMCID: PMC12185836
- DOI: 10.1007/s40268-025-00511-y
Network Meta-Analysis of Pharmacological Therapies for Long-Term Prophylactic Treatment of Patients with Hereditary Angioedema
Abstract
Background and objectives: Several treatments for long-term prophylaxis (LTP) of hereditary angioedema (HAE) are in clinical use, such as garadacimab, lanadelumab, subcutaneous C1 esterase inhibitor (C1INH), and berotralstat. In the absence of head-to-head comparative evidence, indirect comparison methods are needed to compare LTP treatments in patients with HAE. The objective of this analysis was to estimate the comparative efficacy, safety, and impact on quality of life of LTP treatments for patients with HAE through NMAs.
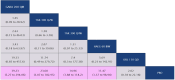
Methods: A systematic literature review was conducted to identify randomized controlled trials (RCTs) investigating LTP treatments in patients (at least 12 years old) with HAE (PROSPERO protocol #CRD42022359207). A network meta-analysis (NMA) feasibility assessment evaluated trial suitability and Bayesian NMAs were conducted for evaluable efficacy, safety, and quality of life (QoL) outcomes.
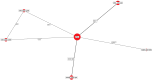
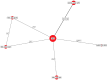
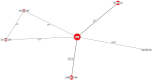
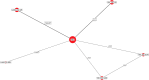
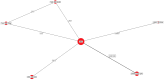
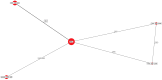
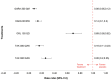
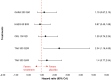
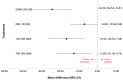
Results: The results of these NMAs show improved efficacy, QoL, and reduced rate of adverse events with garadacimab (200 mg once monthly), lanadelumab (300 mg every two or four weeks), subcutaneous C1INH (60 IU/kg twice weekly), and berotralstat (150 mg once daily) compared to placebo in the treatment of patients with HAE. For the primary outcome of time-normalized number of HAE attacks, garadacimab statistically significantly reduced the rate of attacks compared to lanadelumab Q4W and berotralstat. A similar statistically significant reduction was shown for HAE attacks treated with on-demand treatment. Garadacimab showed statistically significant reduction in the rate of moderate and/or severe HAE attacks compared to lanadelumab Q2W. Garadacimab also showed statistical improvements in change from baseline in AE-QoL total score as compared to berotralstat.
Conclusions: Overall, garadacimab ranked as the most probable effective treatment among all comparators assessed, with lanadelumab Q2W or subcutaneous C1INH ranking second, across most outcomes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This work was supported by CSL Behring. The authors have received no other financial and/or material support for this research or the creation of this work apart from that disclosed. Conflict of interest: John Sears, Yinglei Li, Iris Jacobs, and Neelanjana Ray are employees of CSL Behring, USA. Maebh Kelly is an employee of CSL Behring Ltd. Simona Gavata-Steiger and Chiara Nenci are employees of CSL Behring AG. Ingo Pragst is an employee of CSL Innovation GmbH, Germany. Sarah Walsh, Meaghan Bartlett, Elizabeth M. Salvo-Halloran, and Imtiaz A. Samjoo are employees of EVERSANA, Canada, which was a paid consultant to CSL Behring in connection with the development of this article. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: Openly available data that support the findings of this study are available within the paper and its supplementary information. Data that are not openly available are available upon reasonable request in accordance with the internal CSL Behring data sharing policy. Code availability: Not applicable. Author contributions: All authors participated in the conception and design of the study. SW, MB, EH, and IAS contributed to the collection and analysis of the data. All authors contributed to the interpretation of the data and critically reviewed for the importance of intellectual content for the work. All authors were responsible for drafting or reviewing the manuscript and for providing final approval. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Figures






References
-
- Bernstein J. Severity of hereditary angioedema, prevalence, and diagnostic considerations. AM J Manag Care. 2018;24(14 Suppl.):S292–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

