Therapeutic applications of a novel humanized monoclonal antibody targeting chemokine receptor CCR9 in pancreatic cancer
- PMID: 40434951
- PMCID: PMC12515709
- DOI: 10.1002/1878-0261.70062
Therapeutic applications of a novel humanized monoclonal antibody targeting chemokine receptor CCR9 in pancreatic cancer
Abstract
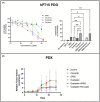
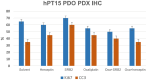
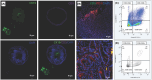
The relative failure of immune checkpoint inhibitors in pancreatic ductal adenocarcinoma (PDAC) despite having a dense, immunosuppressive tumor microenvironment highlights the need to target alternate/escape pathways. We have previously examined C-C chemokine receptor type 9 (CCR9) as a candidate immune checkpoint and developed a targeted, humanized monoclonal antibody (SRB2). Cytotoxicity of SRB2 was evaluated in vitro and in vivo. CCR9 expression on PDAC cells/tissues, immune components of patient-derived organoids (PDOs), and antibody-dependent cell-mediated cytotoxicity were examined. In PANC-1 and MIA PaCa-2 cell lines, we demonstrated highest CCR9 expression; however, no direct cytotoxic effect was observed with SRB2 treatment. In PANC-1 cells, NK cell-mediated cytotoxicity was promoted by SRB2. Dose-dependent SRB2 cytotoxicity was observed in PDAC PDOs. In patient-derived xenograft mouse models, cytotoxicity of SRB2 monotherapy and in combination with oxaliplatin was also shown. In humanized immune-competent mouse models, SRB2 efficacy was similar to other drugs, but two mice in this cohort had complete tumor regression. Our current studies suggest that therapeutic targeting of CCR9 may improve PDAC outcomes, and additional studies are underway to evaluate SRB2 for clinical use.
Keywords: chemokine receptor CCR9; immune checkpoint; pancreatic cancer.
© 2025 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors disclose that DRL, EAC, PG, and LS are employees of SunRock Biopharma and have a financial interest in SRB2 (anti‐CCR9 monoclonal antibody). Other authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

