Dual mechanism of inflammation sensing by the hematopoietic progenitor genome
- PMID: 40435239
- PMCID: PMC12118549
- DOI: 10.1126/sciadv.adv3169
Dual mechanism of inflammation sensing by the hematopoietic progenitor genome
Abstract
Genomes adapt dynamically to alterations in the signaling milieu, including inflammation that transiently or permanently disrupts genome function. Here, we elucidate how a progenitor cell genome senses and responds to inflammation when the developmental and transcriptional regulator GATA2 is limiting, which causes bone marrow failure in humans and mice and predisposes to leukemia in humans. GATA2low murine progenitors are hypersensitive to inflammatory mediators. We discovered that the hematopoietic transcription factor PU.1 conferred transcriptional activation in GATA2low progenitors in response to Interferon-γ and Toll-Like Receptor 1/2 agonists. In a locus-specific manner, inflammation reconfigured genome activity by promoting PU.1 recruitment to chromatin or tuning activity of PU.1-preoccupied chromatin. The recruitment mechanism disproportionately required IKKβ activity. Inflammation-activated genes were enriched in motifs for RUNX factors that cooperate with GATA factors. Contrasting with the GATA2-RUNX1 cooperativity paradigm, GATA2 suppressed and RUNX1 promoted PU.1 mechanisms to endow the progenitor genome with inflammation-sensing capacity.
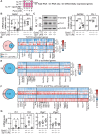
Figures








References
-
- Medzhitov R., The spectrum of inflammatory responses. Science 374, 1070–1075 (2021). - PubMed
-
- Mei Y., Ren K., Liu Y., Ma A., Xia Z., Han X., Li E., Tariq H., Bao H., Xie X., Zou C., Zhang D., Li Z., Dong L., Verma A., Lu X., Abaza Y., Altman J. K., Sukhanova M., Yang J., Ji P., Bone marrow-confined IL-6 signaling mediates the progression of myelodysplastic syndromes to acute myeloid leukemia. J. Clin. Invest. 132, e152673 (2022). - PMC - PubMed
-
- Kleppe M., Kwak M., Koppikar P., Riester M., Keller M., Bastian L., Hricik T., Bhagwat N., McKenney A. S., Papalexi E., Abdel-Wahab O., Rampal R., Marubayashi S., Chen J. J., Romanet V., Fridman J. S., Bromberg J., Teruya-Feldstein J., Murakami M., Radimerski T., Michor F., Fan R., Levine R. L., JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 5, 316–331 (2015). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

