Mitochondrial calcium signaling regulates branched-chain amino acid catabolism in fibrolamellar carcinoma
- PMID: 40435263
- PMCID: PMC12118637
- DOI: 10.1126/sciadv.adu9512
Mitochondrial calcium signaling regulates branched-chain amino acid catabolism in fibrolamellar carcinoma
Abstract
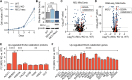
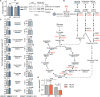
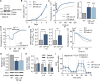
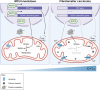
Metabolic adaptations are essential for survival. The mitochondrial calcium uniporter plays a key role in coordinating metabolic homeostasis by regulating mitochondrial metabolic pathways and calcium signaling. However, a comprehensive analysis of uniporter-regulated mitochondrial pathways has remained unexplored. Here, we investigate consequences of uniporter loss and gain of function using uniporter knockout cells and fibrolamellar carcinoma (FLC), which we demonstrate to have elevated mitochondrial calcium levels. We find that branched-chain amino acid (BCAA) catabolism and the urea cycle are uniporter-regulated pathways. Reduced uniporter function boosts expression of BCAA catabolism genes and the urea cycle enzyme ornithine transcarbamylase. In contrast, high uniporter activity in FLC suppresses their expression. This suppression is mediated by the transcription factor KLF15, a master regulator of liver metabolism. Thus, the uniporter plays a central role in FLC-associated metabolic changes, including hyperammonemia. Our study identifies an important role for the uniporter in metabolic adaptation through transcriptional regulation of metabolism and elucidates its importance for BCAA and ammonia metabolism.
Figures




Update of
-
Mitochondrial Calcium Signaling Regulates Branched-Chain Amino Acid Catabolism in Fibrolamellar Carcinoma.bioRxiv [Preprint]. 2024 Nov 30:2024.05.27.596106. doi: 10.1101/2024.05.27.596106. bioRxiv. 2024. Update in: Sci Adv. 2025 May 30;11(22):eadu9512. doi: 10.1126/sciadv.adu9512. PMID: 38853984 Free PMC article. Updated. Preprint.
References
-
- Balderas E., Eberhardt D. R., Lee S., Pleinis J. M., Sommakia S., Balynas A. M., Yin X., Parker M. C., Maguire C. T., Cho S., Szulik M. W., Bakhtina A., Bia R. D., Friederich M. W., Locke T. M., van Hove J. L. K., Drakos S. G., Sancak Y., Tristani-Firouzi M., Franklin S., Rodan A. R., Chaudhuri D., Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during complex I impairment. Nat. Commun. 13, 2769 (2022). - PMC - PubMed
-
- Kwong J. Q., Huo J., Bround M. J., Boyer J. G., Schwanekamp J. A., Ghazal N., Maxwell J. T., Jang Y. C., Khuchua Z., Shi K., Bers D. M., Davis J., Molkentin J. D., The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 3, e121689 (2018). - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

