Infection after CD19 chimeric antigen receptor T-cell therapy for large B-cell lymphoma: real-world analysis from CIBMTR
- PMID: 40435511
- PMCID: PMC12607006
- DOI: 10.1182/bloodadvances.2025016141
Infection after CD19 chimeric antigen receptor T-cell therapy for large B-cell lymphoma: real-world analysis from CIBMTR
Abstract
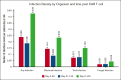
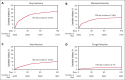
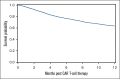
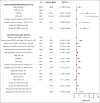
Infection is increasingly recognized as a significant cause of morbidity and mortality in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) receiving CD19 chimeric antigen receptor (CAR) T-cell therapy. The current study analyzed the natural history, risk factors, and outcomes of infection in 3350 patients with R/R LBCL receiving commercial CD19 CAR T-cell therapy (n = 2804 axicabtagene ciloleucel [axi-cel], n = 546 tisagenlecleucel) from December 2017 to June 2022. Infection developed in 834 patients (24.9%) within 100 days after infusion, resulting in an infection density of 0.43 per 100 patient days and a 100-day cumulative incidence of 22%. Bacterial, viral, and fungal infections were recorded in 527 (15.7%), 374 (11.2%), and 108 patients (3.2%), respectively, with corresponding infection densities of 0.23, 0.15, and 0.04 per 100 patient days. After a 24-month median follow-up, 1482 patients (44%) had died, with infection as the primary cause in 173 cases (12%). The 100-day infection-related mortality (IRM) was 1.6% (95% confidence interval, 1.2-2.0). Patients with a Karnofsky performance score of ≤80, infection history before CAR T-cell therapy, axi-cel therapy, severe cytokine release syndrome (grade ≥3), and severe immune effector cell-associated neurotoxicity syndrome (grade ≥3) had increased infection risk. Infections within 100 days were an independent risk factor for inferior overall survival beyond day 100 after CD19 CAR T-cell therapy. In conclusion, study results show a significant incidence of infection and IRM in patients with R/R LBCL treated with CD19 CAR T-cell therapy. Furthermore, results identify patients at a heightened risk of infection, offering insights to guide potential interventions aimed at mitigating infection and improving patient outcomes after CAR T-cell therapy.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: J.H.B. reports honorarium from Kite Pharma, a Gilead company and research funding from Kite Pharma (a Gilead company), Genentech-Roche, Regeneron Pharmaceuticals, Janssen Pharmaceuticals, and Cargo Therapeutics. L.G. received honorarium from Bristol Myers Squibb (BMS). H.G.R. reports consultancy fees from Medexus and Vertex Therapeutics and medical monitoring for CD33CAR T for NMDP (honorary). M.A.K.-D. receives research grant support from BMS, Novartis, and Pharmacyclics, and honorarium from Kite Pharma. S.G. serves on advisory boards for BMS, Kite, Pfizer, and Sanofi. J.-A.H.Y. receives clinical trial reimbursement (through the institution) from AiCuris, AlloVir, Ansun, Basilea, F2G, Gilead, GlaxoSmithKline, Mundipharma (Cidara), Pfizer, Pulmocide, Scynexis, Takeda, and Vedanta. A.S. provides consulting services to Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, Editas Medicine, and BioLineRx; receives research funding from Crispr Therapeutics; serves as a clinical trial site principal investigator (PI) for Crispr Therapeutics, Vertex Pharmaceuticals, Novartis Pharmaceuticals, Magenta Therapeutics, and Beam Therapeutics; receives honoraria from Vindico Medical Education and Blackwood CME; serves as medical monitor for RCI BMT for NMDP; and serves as a data safety monitoring board (DSMB) member for a trial of Children’s Hospital of Philadelphia. G.F. reports advisory board/consultancy for Johnson & Johnson, Knight Pharma, Sanofi, and Astellas; speakers' bureau membership for Novartis, Johnson & Johnson, Takeda, Astellas, Knight, Libbs, Alexion/AstraZeneca, and Sanofi; manuscript writing for Johnson & Johnson, Novartis, and Astellas; research grant from Libbs; and support for meetings and travels from Celgene, Merck Sharp & Dohme (MSD), AstraZeneca, Astellas, and Kite Pharma (a Gilead company). T.J. receives research grant support from CTI Biopharma, Kartos Therapeutics, Incyte, BMS, and TScan Therapeutics, and participated on the advisory boards for BMS, Incyte, AbbVie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios Pharma, Protagonist Therapeutics, Galapagos, TScan Therapeutics, Karyopharm, MorphoSys, and IN8Bio. C.G.K. is an employee of the National Institutes of Health (NIH) and expressed the authors’ own opinions, which do not reflect the views of the NIH, the Department of Health and Human Services (DHHS), or the US government. D.M. reports consulting/advisory role for ADC Therapeutics, Genmab, Genentech (spouse), Daiichi Sankyo (spouse), and AstraZeneca (spouse); research funding for investigator initiated trials from Genentech, Genmab, Karyopharm, and AstraZeneca (spouse); and expert testimony for AstraZeneca. N.S.G. reports research grants from BMS, Cabaletta, and Affimed; serves on data safety monitoring board for Novartis; serves on advisory board for Genentech and Regeneron; and receives research funding from Regeneron and Poseida. M.V.B. received research grants paid to his institution from the NIH/National Cancer Institute (RO1), CNPq (National Council for Scientific and Technological Development), and FAPESP (São Paulo Research Foundation); and honoraria/consulting fees from Takeda, Merck, AbbVie, Janssen/Johnson & Johnson, and Knight. P.V. receives research grant support from Cidara, Mundipharma, Scynexis, Ansun, and F2G via the institution; provides consulting service for Scynexis; and receives honoraria from the MSD Manuals. D.E.Y. is an employee of the NIH and the DHHS and expressed the author’s own opinions, which do not reflect the views of the NIH, the DHHS, or the US government. A.M.B. reports serving as a clinical trial site PI for Crispr Therapeutics, Kite, Autolus, TESSA, and Lyell, and provided consulting services for Kite Pharma (a Gilead company), Autolus, and Legend. T.N. receives clinical trial support to the institution by Novartis and by Karyopharm (drug only supply), and reports consultancy for Alexion. U.G. reports serving as a site PI for Janssen, Takeda, Allogene, Cargo Therapeutics, and BMS; is a speaker's bureau member for Kite; and is an advisory board member for Kyverna and Autolus. M.S. provides consulting service for CVS Caremark and serves on the advisory boards of BMS and A28 Therapeutics. C.E.D. reports honorarium from Omeros, Alexion Pharmaceuticals, and from Alexion for a presentation at the European Society for Blood and Marrow Transplantation. H.S.M. reports advisory board/consultancy for Crispr Therapeutics, BMS, Jazz, Incyte, Sobi, Autolus, and Senti Bioscience, and serves as medical monitor for NMDP/BMT CTN. A.R.H. receives honorarium from Elsevier for the Clinical Overview Chapter. M.-A.P. reports honoraria from Adicet, Allogene, Caribou Biosciences, Celgene, BMS, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite Pharma (a Gilead company), MSD, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, Takeda, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences; has ownership interests in Omeros and OrcaBio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite Pharma (a Gilead company), Miltenyi Biotec, Nektar Therapeutics, and Novartis. R.F.C. serves as a consultant, speaker, or scientific adviser for ADMA Biologics, MSD, Takeda, Shionogi, AiCuris, Astellas, Adagio Therapeutics, Tether, Oxford Immunotec, Pfizer, Moderna, Karius, IntegerBio, Assembly Bio, and Ansun Pharmaceuticals, and received research support (paid to the institution) from MSD, Karius, AiCuris, Ansun Pharmaceuticals, Takeda, Roche/Genentech, Oxford Immunotec, Freestyle, and Eurofins Viracor. J.A.H. reports consultancy for Moderna, AlloVir, Gilead, Takeda, CSL Behring, Karius, Symbio, GeoVax, and Sanofi; reports research funding from Gilead, Takeda, MSD, GeoVax, and Sanofi; and reports significant payments (scientific advisory board) from Karius. M.R. reports being an employee of Iqvia Biotech, a clinical research organization. J.J.A. serves on the advisory committee for AscellaHealth. The remaining authors declare no competing financial interests.
Figures

Comment in
-
After the fog has lifted: infections with CD19 CAR-T therapy.Blood Adv. 2025 Nov 11;9(21):5510-5511. doi: 10.1182/bloodadvances.2025017162. Blood Adv. 2025. PMID: 41171269 No abstract available.
References
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
-
- Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

