Epigenomic insights into the immune regulatory mechanism of GBP4L in poultry
- PMID: 40435797
- PMCID: PMC12159214
- DOI: 10.1016/j.psj.2025.105172
Epigenomic insights into the immune regulatory mechanism of GBP4L in poultry
Abstract
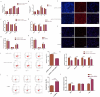
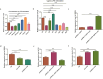
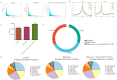
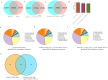
High-temperature environments induce heat stress in poultry, leading to compromised immune function and reduced disease resistance. In the Dexamethasone-induced stress-related immune suppression model in chickens, both transcriptomic and proteomic analyses revealed significantly low expression of GBP4L, highlighting its potential as a key target for combating stress. It is currently unclear whether GBP4L can alleviate the heat stress response in the body. We conducted this study to explore the role of GBP4L in stress resistance. First, to evaluate the role of GBP4L in immune function, we established an inflammatory cell model via Lipopolysaccharide (LPS) treatment and then generated both a pcDNA3.1-EGFP-GBP4L plasmid and a si-GBP4L interference fragment for transfection into HD11 model cells. Next, we exposed 28-day-old Gushi roosters to high heat (32 ± 1 °C) for 4 weeks to establish a heat stress model. We administered a virus carrying pAAV-GBP4L-3FLAG via subcutaneous injection, evaluated immune marker levels and conducted ATAC-seq on spleen tissues to investigate the effect of GBP4L on chromatin accessibility. The results revealed that GBP4L overexpression reduced the expression of proinflammatory factors, promoted the polarization of HD11 cells from the M1 phenotype to the M2 phenotype, reduced the LPS-induced expression of IL-8 (P < 0.05), alleviated inflammation, increased cell proliferation (P < 0.05), and inhibited apoptosis (P < 0.05). In the animal model, increasing the expression of GBP4L alleviated heat stress-induced inflammation, improved blood biochemistry, enhanced immune function in the spleen and bursa of Fabricius, and preserved the structure of the spleen. ATAC-seq revealed that GBP4L reduced chromatin accessibility in the promoter regions of 34 heat stress-induced genes. Furthermore, the expression of SP9 was significantly increased in animals under heat stress but was decreased in animals overexpressing GBP4L under heat stress. In conclusion, GBP4L alleviates heat stress-induced inflammation, enhances immune status, and reduces spleen tissue damage. Mechanistically, GBP4L overexpression may enhance heat stress resistance by altering chromatin spatial structure to regulate SP9 expression. The research findings offer valuable insights into the mechanisms of heat stress resistance in poultry, contributing to the development of strategies to enhance heat tolerance. These results also expand the epigenetic regulation theory of heat tolerance and support the breeding of heat-tolerant chicken varieties.
Keywords: ATAC-seq; Chromatin accessibility; GBP4L; Heat stress; SP9.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Disclosures The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

