CYP1B1 promotes angiogenesis and sunitinib resistance in clear cell renal cell carcinoma via USP5-mediated HIF2α deubiquitination
- PMID: 40435846
- PMCID: PMC12158534
- DOI: 10.1016/j.neo.2025.101186
CYP1B1 promotes angiogenesis and sunitinib resistance in clear cell renal cell carcinoma via USP5-mediated HIF2α deubiquitination
Abstract
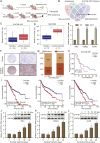
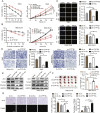
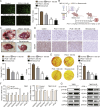
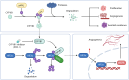
Clear cell renal cell carcinoma (ccRCC) is strongly aetiologically associated with von Hippel‒Lindau (VHL) tumour suppressor gene mutations, which result in constitutive activation of hypoxia-inducible factors and pathological angiogenesis. Although accumulating evidence indicates that antiangiogenic therapies targeting VEGF signalling can prolong the survival of ccRCC patients, the frequent development of therapeutic resistance to tyrosine kinase inhibitors such as sunitinib remains a critical clinical limitation. Through integrated multiomics analyses of sunitinib-resistant cell models, patient-derived xenografts, and clinical specimens, we systematically identified CYP1B1 as a central mediator of treatment resistance. Transcriptomic and genomic profiling revealed that CYP1B1 overexpression in resistant tumours functionally contributes to enhanced angiogenic potential and maintenance of the resistant phenotype. Mechanistic investigations demonstrated that CYP1B1 stabilizes hypoxia-inducible factor 2α (HIF2α) by facilitating USP5-mediated deubiquitination, thereby preventing proteasomal degradation. Notably, we identified VHL as a novel E3 ubiquitin ligase that regulates CYP1B1 turnover; notably, VHL deficiency in ccRCC promotes CYP1B1 protein accumulation by suppressing ubiquitination. These findings establish a feed-forward regulatory axis in which VHL loss-induced CYP1B1 stabilization promotes HIF2α signalling persistence, ultimately driving sunitinib resistance. Our study delineated the CYP1B1-USP5-HIF2α signalling cascade as a critical resistance mechanism and thus reveals a targetable vulnerability in treatment-refractory ccRCC.
Keywords: Angiogenesis; Clear cell renal cell carcinoma; HIF2α; Sunitinib resistance; Ubiquitination.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Yao X., Tan J., Lim K.J., Koh J., Ooi W.F., Li Z., Huang D., Xing M., Chan Y.S., Qu J.Z., Tay S.T., Wijaya G., Lam Y.N., Hong J.H., Lee-Lim A.P., Guan P., Ng M.S.W., He C.Z., Lin J.S., Nandi T., Qamra A., Xu C., Myint S.S., Davies J.O.J., Goh J.Y., Loh G., Tan B.C., Rozen S.G., Yu Q., Tan I.B.H., Cheng C.W.S., Li S., Chang K.T.E., Tan P.H., Silver D.L., Lezhava A., Steger G., Hughes J.R., Teh B.T., Tan P. VHL deficiency drives enhancer activation of oncogenes in clear cell renal cell carcinoma. Cancer Discov. 2017;7:1284–1305. doi: 10.1158/2159-8290.CD-17-0375. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

