Mechanisms of USP18 specificity toward ISG15 revealed by paralog sequence analysis comparison
- PMID: 40436319
- PMCID: PMC12221285
- DOI: 10.1016/j.jbc.2025.110288
Mechanisms of USP18 specificity toward ISG15 revealed by paralog sequence analysis comparison
Abstract
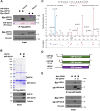
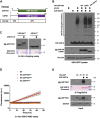
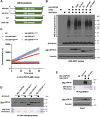
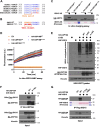
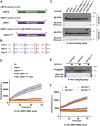
The ubiquitin-like protein ISG15 is activated in response to type 1 interferons, and its conjugation to proteins regulates the response to bacterial and viral infection. Its subsequent deconjugation, which is broadly achieved by the human enzyme USP18, critically controls interferon signaling and the defense against pathogens. However, the molecular determinants underlying USP18 specificity for ISG15 remain elusive. To identify such features, we took advantage of USP18's paralog USP41, which has a strikingly similar catalytic domain and yet lacks deISGylating activity. By performing a comparative sequence analysis coupled with biochemical and enzymatic assays, we identified hallmarks specific to USP18 that are critical for its enzymatic function and ISG15 recognition. Accordingly, AlphaFold-guided analysis suggests that these features mediate USP18-ISG15 interactions, underlining their importance for deISGylating activities. Thus, our results reveal important mechanistic insights into USP18-mediated ISG15 hydrolysis and could inform the development of deISGylase inhibitors relevant to infection and other interferon-related diseases.
Keywords: ISG15; USP18; USP41; alphafold; deISGylating enzymes; deubiquitinating enzymes (DUBs).
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

