Luminescent Liquid-Crystalline J-Aggregate Based on a Columnar Axial Coassembly
- PMID: 40436806
- PMCID: PMC12257545
- DOI: 10.1021/jacs.5c03166
Luminescent Liquid-Crystalline J-Aggregate Based on a Columnar Axial Coassembly
Abstract
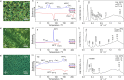
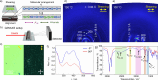
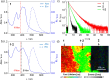
Controlling the self-assembly of dyes is essential for designing functional materials with tailored optical, electronic, and mechanical properties. However, achieving precise structures from two distinct chromophores remains a major challenge in the field, requiring sophisticated strategies to direct their organization at the molecular level. In the present work, we report a novel approach to engineer complex liquid-crystalline (LC) columnar nanostructures through the precise coassembly of two bis-dendronized chromophores: a tris(p-phenyleneethynylene) (TPE) dicarboxylic acid (1) and a tris(p-phenylenevinylene) (TPV) bis(pyridine) (2). TPE 1 forms an unconventional four-stranded orthogonal columnar LC phase via hydrogen bonding between carboxylic acid groups, while TPV 2 adopts a lamellar soft-crystalline phase. Remarkably, their equimolar mixture (1·2) gives rise to an unprecedented two-component columnar liquid crystal. This coassembly is grounded on the complementary hydrogen bonding between pyridine and carboxylic acid groups that leads to the formation of 1D strands composed of alternating molecules of 1 and 2. These strands hierarchically organize by π-π interactions into eight-stranded columnar structures in which the 1/2 molecules are oriented with the transition dipole moments parallel to the columnar axis. This configuration promotes slipped π-π interactions and J-type coupling of the TPE and TPV components, resulting in a fluorescent LC material. This work paves the way for the design of precision multicomponent assemblies, opening exciting avenues for advanced optoelectronic and photonic materials.
Figures


References
-
- Goodby, J. W. ; Collings, P. J. ; Kato, T. ; Tschierske, C. ; Gleeson, H. ; Raynes, P. . Handbook of Liquid Crystals, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2014.
-
- Bushby R. J., Kawata K.. Liquid Crystals That Affected the World: discotic Liquid Crystals. Liq. Crys. 2011;38(11–12):1415–1426. doi: 10.1080/02678292.2011.603262. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

