VCPIP1 facilitates pancreatic adenocarcinoma progression via Hippo/YAP signaling
- PMID: 40436845
- PMCID: PMC12120113
- DOI: 10.1038/s41419-025-07746-2
VCPIP1 facilitates pancreatic adenocarcinoma progression via Hippo/YAP signaling
Abstract
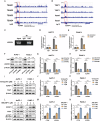
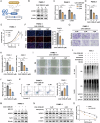
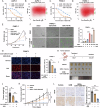
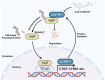
Dysregulation of Hippo signaling is observed in pancreatic adenocarcinoma (PAAD). Moreover, overactivation of YAP is crucial for tumor progression. Although the inhibitory phospho-cascade is functional, the reason for YAP hyperactivation in PAAD remains unclear. Recent studies have revealed that the ubiquitin modification of YAP also plays an important role in the Hippo/YAP axis and cancer progression. To gain a better understanding of the potential mechanisms underlying the ubiquitination and deubiquitination of YAP, we carried out siRNA screening for critical deubiquitinases in PAAD. By using a deubiquitinase (DUB) library, we identified valosin-containing protein-interacting protein 1 (VCPIP1) as an important effector of YAP function and PAAD progression. Inhibition of VCPIP1 hampered PAAD progression via Hippo signaling. Clinical data revealed that VCPIP1 was elevated in PAAD and correlated with poor survival in PAAD patients. Biochemical assays demonstrated that VCPIP1 interacted with YAP, inhibiting K48-linked polyubiquitination and thereby increasing YAP stability. YAP directly binds to the VCPIP1 promoter region, enhancing its transcription in PAAD. Our study revealed a forward feedback loop between VCPIP1 and Hippo signaling in PAAD, indicating that VCPIP1 is a potential therapeutic drug target in PAAD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All procedures were performed in compliance with relevant laws and institutional guidelines and have been approved by the Ethics Committee of Shengjing Hospital, China Medical University. The ethical code number is 2024PS1466K for the use of clinical specimens and 2024PS1486K for animal experiments.
Figures
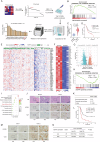
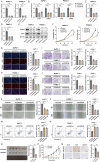

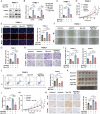

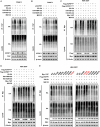
References
MeSH terms
Substances
Grants and funding
- 2023JH2/101700162/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700162/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
- 2023JH2/101700184/Natural Science Foundation of Liaoning Province (Liaoning Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

