BH3 mimetics targeting BCL-XL have efficacy in solid tumors with RB1 loss and replication stress
- PMID: 40436896
- PMCID: PMC12119881
- DOI: 10.1038/s41467-025-60238-x
BH3 mimetics targeting BCL-XL have efficacy in solid tumors with RB1 loss and replication stress
Abstract
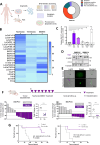
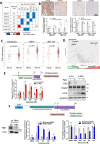
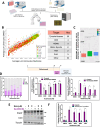
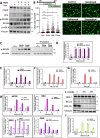
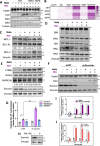
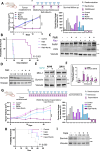
BH3 mimetic drugs that inhibit BCL-2, BCL-XL, or MCL-1 have limited activity in solid tumors. Through assessment of xenograft-derived 3D prostate cancer models and cell lines we find that tumors with RB1 loss are sensitive to BCL-XL inhibition. In parallel, drug screening demonstrates that disruption of nucleotide pools by agents including thymidylate synthase inhibitors sensitizes to BCL-XL inhibition, together indicating that replication stress increases dependence on BCL-XL. Mechanistically we establish that replication stress sensitizes to BCL-XL inhibition through TP53/CDKN1A-dependent suppression of BIRC5 expression. Therapy with a BCL-2/BCL-XL inhibitor (navitoclax) in combination with thymidylate synthase inhibitors (raltitrexed or capecitabine) causes marked and prolonged tumor regression in prostate and breast cancer xenograft models. These findings indicate that BCL-XL inhibitors may be effective as single agents in a subset of solid tumors with RB1 loss, and that pharmacological induction of replication stress may be a broadly applicable approach for sensitizing to BCL-XL inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature435, 677–681 (2005). - PubMed
-
- Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res.68, 3421–3428 (2008). - PubMed
-
- Billard, C. BH3 mimetics: status of the field and new developments. Mol. Cancer Ther.12, 1691–1700 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

