Choroid plexus apocrine secretion shapes CSF proteome during mouse brain development
- PMID: 40437054
- PMCID: PMC12229896
- DOI: 10.1038/s41593-025-01972-9
Choroid plexus apocrine secretion shapes CSF proteome during mouse brain development
Abstract
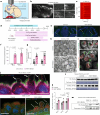
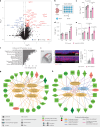
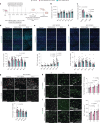
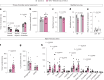
The choroid plexus (ChP) regulates cerebrospinal fluid (CSF) composition, providing essential molecular cues for brain development; yet, embryonic ChP secretory mechanisms remain poorly defined. Here we identify apocrine secretion by embryonic ChP epithelial cells as a key regulator of the CSF proteome and neurodevelopment in male and female mice. We demonstrate that the activation of serotonergic 5-HT2C receptors (by WAY-161503) triggers sustained Ca2+ signaling, driving high-volume apocrine secretion in mouse and human ChP. This secretion alters the CSF proteome, stimulating neural progenitors lining the brain's ventricles and shifting their developmental trajectory. Inducing ChP secretion in utero in mice disrupts neural progenitor dynamics, cerebral cortical architecture and offspring behavior. Additionally, illness or lysergic acid diethylamide exposure during pregnancy provokes coordinated ChP secretion in the mouse embryo. Our findings reveal a fundamental secretory pathway in the ChP that shapes brain development, highlighting how its disruption can have lasting consequences for brain health.
© 2025. The Author(s).
Conflict of interest statement
Competing Interests: The authors declare that they have no competing interests.
Figures








Update of
-
A choroid plexus apocrine secretion mechanism shapes CSF proteome and embryonic brain development.bioRxiv [Preprint]. 2024 Jan 16:2024.01.08.574486. doi: 10.1101/2024.01.08.574486. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Jul;28(7):1446-1459. doi: 10.1038/s41593-025-01972-9. PMID: 38260341 Free PMC article. Updated. Preprint.
References
-
- Silva-Vargas, V., Maldonado-Soto, A. R., Mizrak, D., Codega, P. & Doetsch, F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell19, 643–652 (2016). - PubMed
-
- Damkier, H. H., Brown, P. D. & Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev.93, 1847–1892 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS088566/NS/NINDS NIH HHS/United States
- RF1DA048790/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- P50 HD105351/HD/NICHD NIH HHS/United States
- S10 OD016453/OD/NIH HHS/United States
- R01NS129823/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R35HL145242/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 610670/Simons Foundation
- U54 HD090255/HD/NICHD NIH HHS/United States
- R35 HL145242/HL/NHLBI NIH HHS/United States
- R01NS088566/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P30 CA006516/CA/NCI NIH HHS/United States
- R01 NS129823/NS/NINDS NIH HHS/United States
- P30CA006516/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- RGP0063/2018/Human Frontier Science Program (HFSP)
- RF1 DA048790/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

