The effect of the administration form of antibiotic therapy on the gut microbiome in patients with infected diabetic foot ulcers - DFIATIM trial
- PMID: 40437354
- PMCID: PMC12117690
- DOI: 10.1186/s12866-025-04041-0
The effect of the administration form of antibiotic therapy on the gut microbiome in patients with infected diabetic foot ulcers - DFIATIM trial
Abstract
Background: Diabetic foot infections (DFIs) contribute to the global disability burden. Beta-lactams are the most commonly used antibiotics for treating DFIs. However, the use of antibiotics may lead to disruption of the healthy balance of the gut microbiota, causing dysbiosis.
Methods: Patients with infected diabetic foot ulcers (iDFUs) were treated with two kinds of beta-lactams (amoxicillin/clavulanic acid or ceftazidime) according to microbial sensitivity of causative agents via bolus or continuous administration modes. Changes in the gut microbiome of patients were analyzed. Diabetic patients without iDFUs were used as a control group. 16 S ribosomal RNA gene amplicon sequencing was performed on stool samples collected from participants.
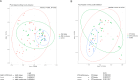
Results: Alpha diversity and beta diversity of gut microbiota of treated patients did not show significant differences between bolus and continuous modes. However, significant differences were observed between gut microbiota diversity of treated patients and control group. PCoA plots showed individualized responses of the patient's gut microbiota to antibiotics at different times using both administration forms associated with the pre-treatment state of microbiota composition. Enterococcus, Sellimonas, and Lachnoclostridium were the common bacterial markers differentially abundant in the gut microbiota of antibiotic-treated patients with iDFUs while Roseburia, Dorea, and Monoglobus were mainly abundant in the gut microbiota of patients without iDFUs. Predicted pathways like "Transporters", "ABC transporters" and "Phosphotranspherase system (PTS)" were upregulated in the gut microbiome of patients treated with bolus regime which may lead to increased intestinal barrier permeability.
Conclusion: The present study reported alterations in gut microbiota composition and functionality and provided the bacterial markers as well as potential metabolic signatures associated with each administration mode in patients with iDFUs, which may be used as a reference set for future studies of the effect of antibiotics administration on the gut microbiome of patients with iDFUs. This study shed light on the importance of understanding the effect of antibiotic administration form on gut microbiome in patients with iDFUs.
Trial registration: The DFIATIM Clinical Trial (Full title: "Rationalisation of ATB therapy in diabetic foot infection and its impact on the intestinal microbiota") is submitted to the European Union Clinical Trials Database under the EudraCT Number: 2019-001997-27. The date of registration is July 17th, 2020.
Keywords: Antibiotics; Beta-lactam; Bolus; Continuous; Diabetes; Diabetic foot infection; Diabetic foot ulcers; Gut microbiota.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The studies involving humans were approved by the ethics committees of the Institute for Clinical and Experimental Medicine and Thomayer University Hospital (Prague, Czech Republic). The DFIATIM Clinical Trial (Full title: “Rationalisation of ATB therapy in diabetic foot infection and its impact on the intestinal microbiota”) is submitted to the European Union Clinical Trials Database under the EudraCT Number: 2019-001997-27. This study was conducted following the local legislation and institutional requirements and in accordance with the ethical principles outlined in the Declaration of Helsinki. All participants provided their written informed consent to participate in this study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Alterations in gut microbiota and inflammatory cytokines after administration of antibiotics in mice.Microbiol Spectr. 2024 Aug 6;12(8):e0309523. doi: 10.1128/spectrum.03095-23. Epub 2024 Jun 20. Microbiol Spectr. 2024. PMID: 38899904 Free PMC article.
-
Impact of Amoxicillin-Clavulanate followed by Autologous Fecal Microbiota Transplantation on Fecal Microbiome Structure and Metabolic Potential.mSphere. 2018 Nov 21;3(6):e00588-18. doi: 10.1128/mSphereDirect.00588-18. mSphere. 2018. PMID: 30463925 Free PMC article.
-
Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers.Gut Microbes. 2017 Jan 2;8(1):17-32. doi: 10.1080/19490976.2016.1267890. Epub 2016 Dec 14. Gut Microbes. 2017. PMID: 27973989 Free PMC article. Clinical Trial.
-
Gut microbiota in epilepsy: How antibiotics induce dysbiosis and influence seizure susceptibility.Microbiol Res. 2025 Sep;298:128225. doi: 10.1016/j.micres.2025.128225. Epub 2025 May 18. Microbiol Res. 2025. PMID: 40398011 Review.
-
The alteration of gut microbiota composition in patients with epilepsy: A systematic review and meta-analysis.Microb Pathog. 2025 Feb;199:107266. doi: 10.1016/j.micpath.2024.107266. Epub 2024 Dec 28. Microb Pathog. 2025. PMID: 39736340
References
-
- Del Core MA, Ahn J, Lewis RB, Raspovic KM, Lalli TAJ, Wukich DK. The evaluation and treatment of diabetic foot ulcers and diabetic foot infections. Foot Ankle Orthop. 2018;3. 10.1177/2473011418788864.
-
- Kim JH. Investigating diabetic foot pathophysiology and amputation prevention strategies through behavioral modification. J Wound Manag Res. 2023;19:167–72. 10.22467/jwmr.2023.02747.
-
- Matheson EM, Bragg SW, Blackwelder RS. Diabetes-related foot infections: diagnosis and treatment. Am Fam Physician. 2021;104:386–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

