Toll-like receptor 9 (TLR9) expression correlates with cell of origin and predicts clinical outcome in diffuse large B-cell lymphoma
- PMID: 40437466
- PMCID: PMC12117956
- DOI: 10.1186/s12885-025-14359-7
Toll-like receptor 9 (TLR9) expression correlates with cell of origin and predicts clinical outcome in diffuse large B-cell lymphoma
Abstract
Background: Biological insights beyond the cell-of-origin (COO) classification can support clinical management in diffuse large B-cell lymphoma (DLBCL). We investigated if Toll-like receptor 9 (TLR9) expression could serve as a prognostic marker in DLBCL.
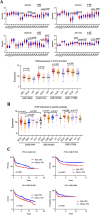
Method: TLR9 gene expression was analysed in four publicly available cohorts (n = 2474), and protein expression was investigated in germinal centre B-cell (GCB) and activated B-cell (ABC) DLBCL cell lines. Next, TLR9 protein expression was analysed in 120 diagnostic samples from R-CHOP-treated patients with relapsed/refractory disease (poor outcome, n = 50) or in complete remission (good outcome, n = 70). Associations were evaluated using logistic regression, estimating odds ratios (OR) and 95% confidence intervals (CI).
Results: TLR9 gene expression was higher in ABC DLBCL compared to GCB DLBCL in external cohorts, and similar results were obtained for protein expression in cell lines. In patient samples, high TLR9 protein expression correlated with non-GCB type (p = 0.003) and poor outcome (p = 0.0016). High TLR9 expression remained associated with poor outcome in multivariable analysis after adjusting for COO and other clinical features (OR = 3.36, 95% CI 1.41-8.04). In exploratory analyses, a decrease of cell growth in ABC cell lines following inhibition of TLR9 activity with ODN4084-F was suggested.
Conclusion: We conclude that TLR9 correlates with ABC/non-GCB phenotype and is a potential predictor of poor prognosis in DLBCL.
Keywords: Biomarker; DLBCL; Prognosis; TLR9.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The regional ethics committee in Stockholm approved the study (Dnr 2015/2028–31/2). The authors confirmed that all experiments using human tissue samples were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from living participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Smedby KEE, Ekberg S, Eloranta S, Enblad G, Jerkeman M, Andersson P-O, Harrysson S. Treatment intensity, timing of relapse and outcome of 713 relapsed/refractory diffuse large B-cell lymphoma (DLBCL) in a population-based setting in Sweden. Blood. 2019;134:4111.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

