Transarterial chemoembolization plus apatinib for unresectable hepatocellular carcinoma: a multicenter, randomized, open-label, phase III trial
- PMID: 40437469
- PMCID: PMC12121135
- DOI: 10.1186/s12916-025-04159-y
Transarterial chemoembolization plus apatinib for unresectable hepatocellular carcinoma: a multicenter, randomized, open-label, phase III trial
Abstract
Background: This study aimed to assess the efficacy and safety of transarterial chemoembolization (TACE) in combination with apatinib (TACE-apatinib) for patients with unresectable hepatocellular carcinoma (HCC).
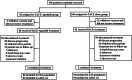
Methods: This study was a multicenter, randomized, open-label, prospective, phase III trial. Patients with unresectable HCC were randomly assigned in a 1:1 ratio to receive either TACE-apatinib or TACE-alone treatment. Patients in the TACE-apatinib group began with a dosage of 500 mg/day of oral apatinib administered 4 days after the first TACE. The primary endpoint of this study was progression-free survival (PFS). The secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), time to untreatable (unTACEable) progression (TTUP), and safety assessment.
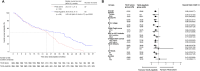
Results: From November 1, 2018 to November 18, 2021, a total of 196 patients were randomly assigned to either the TACE-apatinib (n = 86) or TACE-alone (n = 92) group. The median PFS in the TACE-apatinib group was significantly longer than that of in the TACE-alone group (6.1 months vs. 3.4 months, p < 0.0001). The median OS was significantly prolonged in the TACE-apatinib group compared to the TACE-alone group (28.9 months vs. 24.0 months, p = 0.0005). The median TTUP in the TACE-apatinib group was 26.8 months, which was significantly longer than that of 20.1 months in the TACE-alone group (p = 0.0003). A significantly higher ORR and DCR were observed in the TACE-apatinib group compared to the TACE-alone group (ORR: 58.1% vs. 31.5%, p < 0.001; DCR: 87.2% vs. 69.6%, p = 0.004). Most of the treatment-related adverse events were grades 1-2, and no treatment-related deaths were observed.
Conclusions: Apatinib significantly improved the treatment effects of TACE for patients with unresectable HCC. TACE-apatinib could serve as a promising treatment option for this patient population, offering notable survival benefits while maintaining an acceptable safety profile.
Trial registration: Chinese Clinical Trial Register, No. ChiCTR1800018621.
Keywords: Apatinib; Hepatocellular carcinoma; Progression free survival; Transarterial chemoembolization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This multicenter, randomized, open-label, prospective, phase III trial was carried out in accordance with the principles of the Declaration of Helsinki. The trial protocol was approved by the ethics committee of all participating centers (No. S249-1). All the patients provided a written informed consent before inclusion. This study was registered with the Chinese Clinical Trial Register (Number: ChiCTR1800018621). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. - PubMed
-
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82072041/National Natural Science Foundation of China
- 81873919/National Natural Science Foundation of China
- 2023AFA107/Outstanding Youth Foundation of Hubei Province
- CXPJJH11800001-2018102/Science and Technology Development Foundation Research Fund of Academician Xiaoping Chen for Hepatobiliary and Pancreatic Malignant Tumor
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

