Lecanemab in clinical practice: real-world outcomes in early Alzheimer's disease
- PMID: 40437535
- PMCID: PMC12117801
- DOI: 10.1186/s13195-025-01763-1
Lecanemab in clinical practice: real-world outcomes in early Alzheimer's disease
Abstract
Background: Lecanemab, a monoclonal antibody targeting amyloid beta, has recently been approved for treatment of early-stage Alzheimer's disease (AD), demonstrating amyloid plaque reduction and slowing of cognitive decline in clinical trials. However, real-world data on its efficacy and safety remain limited. The Cognitive Neurology Unit at Tel Aviv Medical Center (TLVMC) established an infrastructure to facilitate advanced treatments for AD, utilising a multidisciplinary approach to patient screening, diagnosis, treatment initiation and follow up.
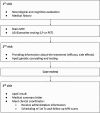
Methods: Lecanemab administration at the TLVMC commenced in November 2023. Patients with biomarker-confirmed early-stage AD were screened via a structured referral system, including neurological evaluations, MRI, lumbar puncture or Amyloid-PET, genetic testing, and multidisciplinary team (MDT) consensus discussions. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) at baseline, six months, and twelve months. Safety monitoring included routine MRI scans for amyloid-related imaging abnormalities (ARIA).
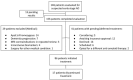
Results: Between July 2023 and January 2025, 169 patients were screened and 86 initiated lecanemab treatment. By January 2025, 53 patients had reached the 6-month follow-up date. In the intention-to-treat (ITT) population, MMSE scores declined significantly over 6 months (F(1, 45.13) = 7.41, p =.009). Subgroup analysis revealed a significant decline in younger patients (n = 31; F(1, 24.67) = 8.06, p =.009), but not in older patients (n = 22; F(1, 19.25) = 0.67, p =.424). At 12 months, 31 patients had reached follow-up, with no significant change in MMSE scores observed (F(1, 17.18) = 2.49, p =.133). Age subgroup analysis was not performed at 12 months due to limited sample size. No significant correlations were found between baseline biomarkers and cognitive change. ARIA occurred in 18.6% of patients, mostly asymptomatic. One patient experienced symptomatic ARIA, required hospitalization with intravenous treatment, and discontinued therapy. A mixed-effects model showed no significant effect of ARIA on MMSE change (p =.264) and no interaction with time (p =.433). Infusion-related reactions occurred in 22.1%, all mild and transient. Treatment was discontinued in 19.8% of patients due to ARIA, financial barriers, comorbidities, or personal preference.
Conclusions: This real-world analysis demonstrates the feasibility and safety of Lecanemab administration for early-stage AD within a tertiary hospital setting. Establishing dedicated infrastructure enabled streamlined patient evaluations and treatment. The findings suggest a differential response across age groups, consistent with clinical trial data. Continued longitudinal follow-up is needed to assess long-term efficacy and safety.
Keywords: Alzheimer's disease; Anti-amyloid therapy; Lecanemab; Real-world data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the IRB committee of TLVMC, approval number 850 − 16. All participants provided written informed consent before inclusion in the study, in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. This manuscript does not contain any individual person’s data in any form. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical