Targeted limbic self-neuromodulation for alleviating central sensitization symptoms in fibromyalgia
- PMID: 40437546
- PMCID: PMC12121213
- DOI: 10.1186/s12916-025-04138-3
Targeted limbic self-neuromodulation for alleviating central sensitization symptoms in fibromyalgia
Abstract
Background: Fibromyalgia (FM), involving somatic, cognitive, and affective domains is often regarded as a hallmark central sensitization syndrome. Despite limited current therapeutic options, emerging understanding of its neural underpinnings offers the potential of applying novel neuromodulation strategies. Specifically, limbic dysregulation underlying abnormalities in pain modulation and somatic-affective processing, has been shown to play a key role in FM. Here, we assessed the long-term efficacy of targeted limbic self-neuromodulation for improving clinical disease burden in FM.
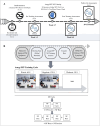
Methods: Forty-seven patients with FM participated in a double-blind, randomized, dual-control study employing a novel specialized neurofeedback probe representing amygdala activity. Patients underwent 10 sessions of either genuine neurofeedback training (NFT = 21), or sham neurofeedback training (NFS = 13), or treatment as usual (TAU = 13). Disease severity and symptom burden were assessed using the Symptom Severity Score (SSS), along with other questionnaires administered before and after treatment. A clinical follow-up was performed 10-12 months post-intervention.
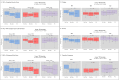
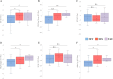
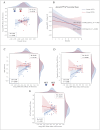
Results: NFT led to a significant immediate and long-term reduction in the SSS (F(2,40) = 7.32, p = 0.00, ηp2 = 0.27) and the Fibromyalgia Impact Questionnaire (FIQ) (F(2,40) = 9.85, p = 0.00, ηp2 = 0.33), alongside multidomain short- and long-term clinical benefits. NFS resulted in a long-term reduction in pain but did not affect other disease measures or overall disease burden. The TAU group showed no clinical improvements.
Conclusions: Our findings support the intimate involvement of limbic brain areas in the pathophysiology of FM and suggest that targeted neuromodulation offers a novel, mechanism-based approach for managing multidomain symptoms in FM.
Trial registration: This study was preregistered with the National Institutes of Health (NIH).
Registration number: NCT02146495. Name of trial registry: Targeted Limbic Self-modulation as a Potential Treatment for Patients Suffering From Fibromyalgia https://clinicaltrials.gov/study/NCT02146495 .
Keywords: Brain-based therapy; Chronic pain relief; FMRI-informed EEG model; Fibromyalgia management; Non-invasive intervention.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol received approval from the Institutional Ethical Review Board of Tel Aviv Sourasky Medical Center (TASMC), reference number 0044–14-TLV. Written informed consent was obtained from all participants prior to their enrollment in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

