Bridging systemic metabolic dysfunction and Alzheimer's disease: the liver interface
- PMID: 40437610
- PMCID: PMC12121119
- DOI: 10.1186/s13024-025-00849-6
Bridging systemic metabolic dysfunction and Alzheimer's disease: the liver interface
Abstract
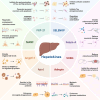
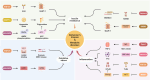
Alzheimer's disease (AD) is increasingly recognized as a systemic disorder with a substantial metabolic disorder component, where the liver significantly impacts the brain via the liver-brain axis. Key mechanisms include the liver's role in clearing peripheral β-amyloid (Aβ), the influence of hepatic enzymes and metabolites on cognitive decline, and the systemic effects of metabolic disorders on AD progression. Hepatokines, liver-secreted proteins including fibroblast growth factor (FGF)-21, selenoprotein P (SELENOP), Fetuin-A, Midbrain astrocyte-derived neurotrophic factor (MANF), apolipoprotein J (ApoJ), sex hormone-binding globulin (SHBG), Adropin and Angiopoietin-like protein 3 (ANGPTL3), could regulate insulin sensitivity, lipid metabolism, oxidative stress, immune responses, and neurotrophic support. These pathways are closely linked to core AD pathologies, including Aβ aggregation, tau hyperphosphorylation, neuroinflammation, oxidative stress and mitochondrial dysfunction. Lifestyle interventions, including exercise and dietary modifications, that regulate hepatokines expression may offer novel preventive and therapeutic strategies for AD. This review synthesizes current knowledge on the liver-brain crosstalk in AD, emphasizing the mechanistic role of liver in bridging metabolic dysfunction with neurodegeneration and underscores the diagnostic and therapeutic potential of hepatokines in addressing AD's complex pathology.
Keywords: Alzheimer’s disease; Hepatokines; Liver; Metabolic disorders; Metabolism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Nedelec T, Couvy-Duchesne B, Monnet F, Daly T, Ansart M, Gantzer L, Lekens B, Epelbaum S, Dufouil C, Durrleman S. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digit Health. 2022;4:e169–78. - PubMed
-
- Berná G, Romero-Gomez M. The role of nutrition in non-alcoholic fatty liver disease: pathophysiology and management. Liver International: Official J Int Association Study Liver. 2020;40(Suppl 1):102–8. - PubMed
-
- Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, Hagihara Y, Taniki N, Kohno K, Shibata S, et al. The liver-brain-gut neural Arc maintains the T(reg) cell niche in the gut. Nature. 2020;585:591–6. - PubMed
Publication types
MeSH terms
Grants and funding
- No.82371427/National Natural Science Foundation of China
- No.82401656/National Natural Science Foundation of China
- CSTB2024NSCQ-MSX0332/Natural Science Foundation of Chongqing Municipality
- CSTB2024NSCQ-MSX0323/Natural Science Foundation of Chongqing Municipality
- CSTB2023NSCQ-BHX0018/Natural Science Foundation of Chongqing Municipality
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

