Intermittent Supplementation With Fisetin Improves Physical Function and Decreases Cellular Senescence in Skeletal Muscle With Aging: A Comparison to Genetic Clearance of Senescent Cells and Synthetic Senolytic Approaches
- PMID: 40437670
- PMCID: PMC12341784
- DOI: 10.1111/acel.70114
Intermittent Supplementation With Fisetin Improves Physical Function and Decreases Cellular Senescence in Skeletal Muscle With Aging: A Comparison to Genetic Clearance of Senescent Cells and Synthetic Senolytic Approaches
Abstract
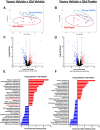
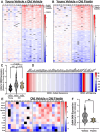
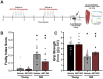
Excess cellular senescence contributes to age-related increases in frailty and reductions in skeletal muscle strength. In the present study, we determined the efficacy of oral intermittent treatment (1 week on-2 weeks off-1 week on) with the natural flavonoid senolytic fisetin to improve frailty and grip strength in old mice. Further, the effects of fisetin on physical function were evaluated in young mice. We performed bulk RNA sequencing of quadricep skeletal muscle to determine the cell senescence-related signaling pathways modulated by fisetin. We also assessed the relative effects of fisetin on frailty and grip strength with aging in comparison with two other well-established approaches for the removal of senescent cells: (1) genetic-based clearance of excess senescent cells in old p16-3MR mice, a model that allows for clearance of p16-positive (p16+) senescent cells, and (2) oral intermittent treatment with the synthetic pharmacological senolytic ABT-263 in old mice. We found that fisetin mitigated the adverse changes in frailty and grip strength with aging. Fisetin had no effects in young mice. The improvements in frailty and grip strength in old mice were accompanied by favorable modulation of the skeletal muscle transcriptome, including lower abundance of cellular senescence-related genes (e.g., Cdkn1a and Ddit4). Improvements in frailty and grip strength with fisetin were comparable to those observed with genetic-based clearance of excess p16+ senescent cells and treatment with ABT-263. Taken together, our findings provide proof-of-concept support for fisetin as a senolytic strategy to improve physical function with aging.
Keywords: flavonoid; motor function; natural senolytic; senescence associated secretory phenotype; skeletal muscle senescence; transcriptome.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures



Similar articles
-
At the Nexus Between Epigenetics and Senescence: The Effects of Senolytic (BI01) Administration on DNA Methylation Clock Age and the Methylome in Aged and Regenerated Skeletal Muscle.Aging Cell. 2025 Jul;24(7):e70068. doi: 10.1111/acel.70068. Epub 2025 Apr 21. Aging Cell. 2025. PMID: 40259517 Free PMC article.
-
Identification of a senescence-associated transcriptional program in skeletal muscle of cachectic pancreatic-tumor-bearing mice.Am J Physiol Cell Physiol. 2025 Apr 1;328(4):C1125-C1134. doi: 10.1152/ajpcell.00816.2024. Epub 2025 Feb 24. Am J Physiol Cell Physiol. 2025. PMID: 39993009 Free PMC article.
-
Apoptotic priming in senescence predicts specific senolysis by quantitative analysis of mitochondrial dependencies.Cell Death Differ. 2025 May;32(5):802-817. doi: 10.1038/s41418-024-01431-1. Epub 2025 Jan 6. Cell Death Differ. 2025. PMID: 39762561
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
In vitro senescence and senolytic functional assays.Biomater Sci. 2025 Jun 25;13(13):3509-3531. doi: 10.1039/d4bm01684j. Biomater Sci. 2025. PMID: 40375674 Review.
Cited by
-
Immunosenescence and the Geriatric Giants: Molecular Insights into Aging and Healthspan.Med Sci (Basel). 2025 Jul 28;13(3):100. doi: 10.3390/medsci13030100. Med Sci (Basel). 2025. PMID: 40843723 Free PMC article. Review.
References
-
- Agergaard, J. , Zillmer M. C. F., Castro‐Mejía J. L., et al. 2021. “Impaired Skeletal Muscle Hypertrophy Signaling and Amino Acid Deprivation Response in Apoe Knockout Mice With an Unhealthy Lipoprotein Distribution.” Scientific Reports 11, no. 1: 16423. 10.1038/s41598-021-96000-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

