Stereoselectivity of Aminoacyl-RNA Loop-Closing Ligation
- PMID: 40437938
- PMCID: PMC12164329
- DOI: 10.1021/jacs.4c16905
Stereoselectivity of Aminoacyl-RNA Loop-Closing Ligation
Abstract
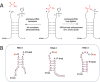
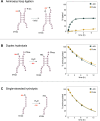
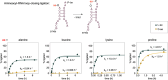
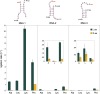
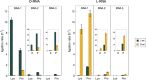
The origin of amino acid homochirality remains an unresolved question in the origin of life. The requirement of enantiopure nucleotides for nonenzymatic RNA copying strongly suggests that the homochirality of nucleotides and RNA arose early. However, this leaves open the question of whether and how homochiral RNA subsequently imposes biological homochirality on other metabolites, including amino acids. Previous studies have reported moderate stereoselectivity for various aminoacyl-RNA transfer reactions. Here, we examine aminoacyl-RNA loop-closing ligation, a reaction that "captures" aminoacylated RNA in a stable phosphoramidate product, such that the amino acid bridges two nucleotides in the RNA backbone. We find that the rate of this reaction is much higher for RNA aminoacylated with L-amino acids than for RNA aminoacylated with D-amino acids. We present an RNA sequence that nearly exclusively captures L-amino acids in loop-closing ligation. Finally, we demonstrate that ligation of aminoacyl-L-RNA results in an inverse stereoselectivity for D-amino acids. The observed stereochemical link between D-RNA and L-amino acids in the synthesis of RNA stem-loops containing bridging amino acids constitutes a stereoselective structure-building process. We suggest that this process led to a selection for the evolution of aminoacyl-RNA synthetase ribozymes that were selective for L-amino acids, thereby setting the stage for the subsequent evolution of homochiral peptides and, ultimately, protein synthesis.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

