Influence of α-Linolenic Acid on the Intestinal Barrier Integrity and Intestinal Antioxidant Status in Broilers
- PMID: 40438093
- PMCID: PMC12117540
- DOI: 10.1002/fsn3.70271
Influence of α-Linolenic Acid on the Intestinal Barrier Integrity and Intestinal Antioxidant Status in Broilers
Abstract
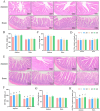
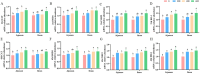
This study aimed to investigate the beneficial effects of α-linolenic acid (ALA) on intestinal barrier function and antioxidant status in broilers, along with the associated molecular mechanisms. 320 one-day-old Arbor Acres broilers were randomly divided into four groups, each with eight replicates, and fed diets with 0 (control), 200, 400, and 600 mg of ALA/kg for 42 days. ALA supplementation did not significantly affect the broilers' overall growth performance. Supplementing diets with 400 and 600 mg/kg of ALA significantly enhanced (p < 0.05) jejunal and ileal villus height, the jejunal villus height to crypt depth ratio, and ileal mRNA expression and protein levels of Zonula occludens-1 (ZO-1) and occludin in broilers on Day 42. Broilers fed diets containing 600 mg/kg of ALA exhibited significantly increased (p < 0.05) serum catalase (CAT) activity, total antioxidant capacity (T-AOC), and jejunal and ileal activities of CAT and total superoxide dismutase (T-SOD), alongside reduced malondialdehyde (MDA) concentrations in serum, jejunum, and ileum on Days 21 and 42, compared to the control group. Supplementing 600 mg/kg of ALA significantly increased (p < 0.05) the mRNA expressions of CAT, SOD1, NRF2, and HO-1, along with the protein levels of cytoplasmic and nuclear NRF2 and HO-1 in the jejunum and ileum on Days 21 and 42. These findings demonstrate the protective effects of ALA in improving intestinal health in broilers. The underlying mechanisms may involve enhancing intestinal barrier integrity by increasing tight junction protein abundance and boosting intestinal antioxidant capacity by elevating antioxidant enzyme activity and activating the NRF2 pathway. In conclusion, our results showed that 600 mg/kg of ALA was identified as the optimal concentration for improving intestinal barrier function and antioxidant status in broilers, highlighting its potential for protecting intestinal health through ALA-based interventions.
Keywords: broiler; growth performance; intestinal antioxidant status; intestinal barrier function; α‐linolenic acid.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Chitosan oligosaccharide improves intestinal function by promoting intestinal development, alleviating intestinal inflammatory response, and enhancing antioxidant capacity in broilers aged d 1 to 14.Poult Sci. 2024 Feb;103(2):103381. doi: 10.1016/j.psj.2023.103381. Epub 2023 Dec 15. Poult Sci. 2024. PMID: 38157786 Free PMC article.
-
Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers.J Anim Sci. 2019 Apr 3;97(4):1679-1692. doi: 10.1093/jas/skz078. J Anim Sci. 2019. PMID: 30789669 Free PMC article.
-
Effect of Tannic Acid on Antioxidant Function, Immunity, and Intestinal Barrier of Broilers Co-Infected with Coccidia and Clostridium perfringens.Animals (Basel). 2024 Mar 19;14(6):955. doi: 10.3390/ani14060955. Animals (Basel). 2024. PMID: 38540053 Free PMC article.
-
Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers.Poult Sci. 2023 Mar;102(3):102408. doi: 10.1016/j.psj.2022.102408. Epub 2022 Dec 9. Poult Sci. 2023. PMID: 36584416 Free PMC article.
-
Effects of different levels of modified palygorskite supplementation on the growth performance, immunity, oxidative status and intestinal integrity and barrier function of broilers.J Anim Physiol Anim Nutr (Berl). 2018 Dec;102(6):1574-1584. doi: 10.1111/jpn.12974. Epub 2018 Aug 16. J Anim Physiol Anim Nutr (Berl). 2018. PMID: 30113108
References
-
- Broom, L. J. , and Kogut M. H.. 2018. “The Role of the Gut Microbiome in Shaping the Immune System of Chickens.” Veterinary Immunology and Immunopathology 204: 44–51. - PubMed
-
- Caligiuri, S. P. B. , Parikh M., Stamenkovic A., Pierce G. N., and Aukema H. M.. 2017. “Dietary Modulation of Oxylipins in Cardiovascular Disease and Aging.” American Journal of Physiology. Heart and Circulatory Physiology 313: H903–H918. - PubMed
-
- Carroll, A. E. , and Hwang D. H.. 1980. “Decreased Formation of Porstaglandins Derived From Arachidonic Acid by Dietary Linolenate in Rats.” American Journal of Clinical Nutrition 33: 590–597. - PubMed
-
- Chairakaki, A. D. , and Greece T.. 2009. “The Role of Intestinal Microbial/Immune Cell Interactions in Patients With Inflammatory Bowel Disease.” Annals of Gastroenterology 22: 239–241.
-
- Chen, F. , Zhang H., Du E. C., Fan Q. W., Zhao N., and Wei J.. 2021. “Supplemental Magnolol or Honokiol Attenuates Adverse Effects in Broilers Infected With Salmonella Pullorum by Modulating Mucosal Gene Expression and the Gut Microbiota.” Journal of Animal Science and Biotechnology 12: 87. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

