Compensatory mechanisms in γδ T cell-deficient chickens following Salmonella infection
- PMID: 40438105
- PMCID: PMC12117349
- DOI: 10.3389/fimmu.2025.1576766
Compensatory mechanisms in γδ T cell-deficient chickens following Salmonella infection
Abstract
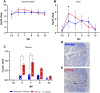
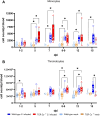
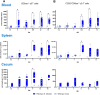
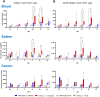
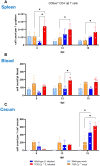
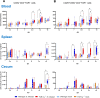
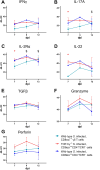
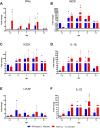
Avian γδ T lymphocytes are highly abundant in the intestinal mucosa and play a critical role in immune defense against infectious diseases in chickens. However, their specific contributions to infection control remain poorly understood. To investigate the role of γδ T cells and their possible compensation, we studied wild-type and γδ T cell knockout chickens following infection with Salmonella Enteritidis. Bacterial loads in the liver, cecal content, and cecal wall were quantified. Immune cell populations in blood, spleen, and cecum were analyzed using flow cytometry. Immune gene transcription in sorted γδ (TCR1+) and TCR1- cell subsets as well as cecal tissue was measured by RT-qPCR. Strikingly, chickens lacking γδ T cells had significantly higher bacterial loads in the liver and more extensive Salmonella invasion in the cecal wall during the early stages of infection compared to wild-type birds. In blood, infected γδ T cell knockout chickens displayed a significantly increased percentage of CD25+ NK-like cells. In both blood and tissue, infected wild-type chickens demonstrated an increased absolute number of CD8αα+hi γδ T cells (CD4-). Conversely, γδ T cell knockout chickens exhibited an augmented cell count of a CD8αα+hiCD4-TCR1- cell population after infection, which might include αβ T cells. At 7 days post infection (dpi), gene expression analysis revealed elevated transcription of the activation marker IL-2Rα and proinflammatory cytokines (IL-17A, IFN-γ) in CD8αα+hiCD4-TCR1- cells from γδ T cell knockout chickens compared to CD8αα+hi γδ T cells from wild-type birds. By 12 dpi, these differences diminished as transcription levels increased in γδ T cells of wild-type animals. Our findings demonstrate that γδ T cells play a role in early immune protection against Salmonella Enteritidis infection in chickens. In later stages of the infection, the γδ T cells and their functions appear to be replaced by other cells.
Keywords: CD8α T cells; Salmonella; chickens; compensation; knockout; γδ T cells.
Copyright © 2025 Tetzlaff, Methner, von Heyl, Menge, Schusser and Berndt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Circulating gamma delta T cells in response to Salmonella enterica serovar enteritidis exposure in chickens.Infect Immun. 2006 Jul;74(7):3967-78. doi: 10.1128/IAI.01128-05. Infect Immun. 2006. PMID: 16790770 Free PMC article.
-
Gamma/delta T cell response of chickens after oral administration of attenuated and non-attenuated Salmonella typhimurium strains.Vet Immunol Immunopathol. 2001 Jan 26;78(2):143-61. doi: 10.1016/s0165-2427(00)00264-6. Vet Immunol Immunopathol. 2001. PMID: 11182154
-
Characterization of avian γδ T-cell subsets after Salmonella enterica serovar Typhimurium infection of chicks.Infect Immun. 2011 Feb;79(2):822-9. doi: 10.1128/IAI.00788-10. Epub 2010 Nov 15. Infect Immun. 2011. PMID: 21078853 Free PMC article.
-
Avian CD25(+) gamma/delta (γδ) T cells after Salmonella exposure.Vet Immunol Immunopathol. 2015 Nov 15;168(1-2):14-8. doi: 10.1016/j.vetimm.2015.09.010. Epub 2015 Sep 28. Vet Immunol Immunopathol. 2015. PMID: 26553561
-
AMPK and mTOR: sensors and regulators of immunometabolic changes during Salmonella infection in the chicken.Poult Sci. 2016 Feb;95(2):345-53. doi: 10.3382/ps/pev349. Epub 2015 Dec 25. Poult Sci. 2016. PMID: 26706353 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

