Sex disparities in hepatocellular carcinoma immunotherapy: hormonal and genetic influences on treatment efficacy
- PMID: 40438106
- PMCID: PMC12116488
- DOI: 10.3389/fimmu.2025.1607374
Sex disparities in hepatocellular carcinoma immunotherapy: hormonal and genetic influences on treatment efficacy
Abstract
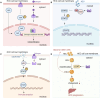
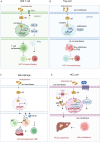
Hepatocellular carcinoma (HCC) is a highly aggressive liver cancer with a rising incidence globally. Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized HCC treatment, yet response rates remain variable. Sex-based disparities in immunotherapy efficacy have become increasingly recognized as important factors influencing treatment outcomes in HCC. This review examines the role of biological sex in HCC progression and immunotherapy responses. It discusses the epidemiology of sex differences in HCC incidence, prognosis, and therapeutic outcomes, highlighting the impact of sex hormones, such as estrogen and testosterone, on immune system function and tumor biology. Estrogen's protective effects, including enhanced T cell activation and improved immune surveillance, contribute to better treatment responses in females, while testosterone's immunosuppressive effects lead to poorer outcomes in males. The review also explores the influence of the tumor microenvironment, including immune cell composition and macrophage polarization, on treatment efficacy. Emerging evidence suggests that sex-specific factors, including hormonal status, should be considered in clinical trials and personalized treatment strategies. By addressing these disparities, tailored immunotherapeutic approaches could optimize efficacy and minimize toxicity in both male and female HCC patients, ultimately improving overall outcomes.
Keywords: estrogen; hepatocellular carcinoma; hormone; immunotherapy; sex disparities.
Copyright © 2025 Song, Sun, Ren, Wang and Xian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
m6A epitranscriptomic modification in hepatocellular carcinoma: implications for the tumor microenvironment and immunotherapy.Front Immunol. 2025 Feb 17;16:1538658. doi: 10.3389/fimmu.2025.1538658. eCollection 2025. Front Immunol. 2025. PMID: 40034695 Free PMC article. Review.
-
DNA Damage Repair Status Predicts Opposite Clinical Prognosis Immunotherapy and Non-Immunotherapy in Hepatocellular Carcinoma.Front Immunol. 2021 Jul 15;12:676922. doi: 10.3389/fimmu.2021.676922. eCollection 2021. Front Immunol. 2021. PMID: 34335575 Free PMC article.
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives.J Hematol Oncol. 2024 Apr 29;17(1):25. doi: 10.1186/s13045-024-01549-2. J Hematol Oncol. 2024. PMID: 38679698 Free PMC article. Review.
-
Immune microenvironment and immunotherapy in hepatocellular carcinoma: mechanisms and advances.Front Immunol. 2025 Apr 2;16:1581098. doi: 10.3389/fimmu.2025.1581098. eCollection 2025. Front Immunol. 2025. PMID: 40242773 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

