Nucleic acid vaccines: innovations, efficacy, and applications in at-risk populations
- PMID: 40438110
- PMCID: PMC12116436
- DOI: 10.3389/fimmu.2025.1584876
Nucleic acid vaccines: innovations, efficacy, and applications in at-risk populations
Abstract
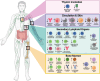
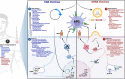
For more than two centuries, the field of vaccine development has progressed through the adaptation of novel platforms in parallel with technological developments. Building off the advantages and shortcomings of first and second-generation vaccine platforms, the advent of third-generation nucleic acid vaccines has enabled new approaches to tackle emerging infectious diseases, cancers, and pathogens where vaccines remain unavailable. Unlike traditional vaccine platforms, nucleic acid vaccines offer several new advantages, including their lower cost and rapid production, which was widely demonstrated during the COVID-19 pandemic. Beyond production, DNA and mRNA vaccines can elicit unique and targeted responses through specialized design and delivery approaches. Considering the growth of nucleic acid vaccine research over the past two decades, the evaluation of their efficacy in at-risk populations is paramount for refining and improving vaccine design. Importantly, the aging population represents a significant portion of individuals highly susceptible to infection and disease. This review seeks to outline the major impairments in vaccine-induced responses due to aging that may be targeted for improvement with design and delivery components encompassing mRNA and DNA vaccine formulations. Results of pre-clinical and clinical applications of these vaccines in aged animal models and humans will also be evaluated to outline current successes and limitations observed in these platforms.
Keywords: DNA vaccine; aging; immunosenescence; mRNA vaccine; nucleic acid vaccines.
Copyright © 2025 Konopka, Edgerton and Kutzler.
Conflict of interest statement
Author MK receives grant funding, participates in industry collaborations funded through sponsored research agreements. MK holds patent licensure through Drexel University and Inovio Pharmaceuticals for molecular adjuvants and DNA vaccine antigens. The remaining authors that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Advances in nucleic acid-based cancer vaccines.J Biomed Sci. 2025 Jan 21;32(1):10. doi: 10.1186/s12929-024-01102-w. J Biomed Sci. 2025. PMID: 39833784 Free PMC article. Review.
-
Application of Traditional Vaccine Development Strategies to SARS-CoV-2.mSystems. 2023 Apr 27;8(2):e0092722. doi: 10.1128/msystems.00927-22. Epub 2023 Mar 2. mSystems. 2023. PMID: 36861991 Free PMC article. Review.
-
Nucleic acid-based vaccine platforms against the coronavirus disease 19 (COVID-19).Arch Microbiol. 2023 Mar 30;205(4):150. doi: 10.1007/s00203-023-03480-5. Arch Microbiol. 2023. PMID: 36995507 Free PMC article. Review.
-
mRNA Vaccine: How to Meet the Challenge of SARS-CoV-2.Front Immunol. 2022 Jan 21;12:821538. doi: 10.3389/fimmu.2021.821538. eCollection 2021. Front Immunol. 2022. PMID: 35126377 Free PMC article. Review.
-
Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses.Med Sci (Basel). 2024 May 22;12(2):28. doi: 10.3390/medsci12020028. Med Sci (Basel). 2024. PMID: 38804384 Free PMC article. Review.
References
-
- Beleche T, Sertkaya A, Knope C, Ertis MD, Berlind A. Preventive Vaccine Development Final. Washington, DC: Office of the Assistant Secretary for Planning and Evaluation; (2022). - PubMed
-
- Wilmoth JR, Bas D, Mukherjee S, Hanif N. World social report 2023: Leaving no one behind in an ageing world: UN. New York, NY: United Nations; (2023).
-
- Bureau PR. 2024 World Population Data Sheet prb.org: Population Reference Bureau (2024). Available online at: https://2024-wpds.prb.org/ (Accessed February 15, 2025).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

