Urinary Myeloid Bodies as a Biomarker for Early Diagnosis and Monitoring of Enzyme Replacement Therapy in Fabry Disease
- PMID: 40438135
- PMCID: PMC12119078
- DOI: 10.1159/000545604
Urinary Myeloid Bodies as a Biomarker for Early Diagnosis and Monitoring of Enzyme Replacement Therapy in Fabry Disease
Abstract
Introduction: The prevalence of urinary myeloid bodies in Fabry disease patients and their correlation with renal involvement remains unclear.
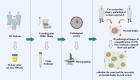
Methods: This single-center, retrospective study included 25 patients with Fabry disease and 27 controls. We analyzed 24-h urine samples for the presence of urinary myeloid bodies and evaluated clinical data, including serum creatinine, estimated glomerular filtration rate (eGFR), 24-h urinary protein levels, α-Gal A, and Lyso-GL-3. Seven Fabry patients underwent analysis of urine samples before and after 1 year of enzyme replacement therapy (ERT).
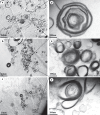
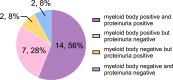
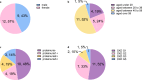
Results: Urinary myeloid bodies were detected in 84% of Fabry patients (21 out of 25), with no significant gender differences. None of the healthy controls or patients with other renal disease patients had urinary myeloid bodies. Among the Fabry patients with myeloid bodies, 48% had no proteinuria, and 52% were in CKD1 stage G1. Furthermore, urinary myeloid bodies were detected in 4 patients under the age of 20, despite the absence of or only minimal proteinuria, and these patients all exhibited a substantial number of myeloid bodies. After 1 year of ERT, significant reductions in both the count (p = 0.043) and area ratio (p = 0.028) of myeloid bodies were observed.
Conclusion: Urinary myeloid bodies are specific to Fabry disease and are associated with early renal injury, even in the absence of proteinuria. These findings suggest that urinary myeloid bodies may serve as a noninvasive biomarker for the early diagnosis of Fabry disease and for monitoring the efficacy of ERT.
Keywords: Fabry disease; Hereditary disease; Urinary myeloid bodies.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
All the authors declared no competing interests.
Figures

Similar articles
-
Long-term enzyme replacement therapy is associated with reduced proteinuria and preserved proximal tubular function in women with Fabry disease.Nephrol Dial Transplant. 2014 Mar;29(3):619-25. doi: 10.1093/ndt/gft452. Epub 2013 Nov 8. Nephrol Dial Transplant. 2014. PMID: 24215016
-
Increased urinary CD80 excretion and podocyturia in Fabry disease.J Transl Med. 2016 Oct 13;14(1):289. doi: 10.1186/s12967-016-1049-8. J Transl Med. 2016. PMID: 27733175 Free PMC article.
-
Urinary mulberry bodies as a potential biomarker for early diagnosis and efficacy assessment of enzyme replacement therapy in Fabry nephropathy.Nephrol Dial Transplant. 2021 Dec 31;37(1):53-62. doi: 10.1093/ndt/gfaa298. Nephrol Dial Transplant. 2021. PMID: 33367839
-
The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts.Mol Genet Metab Rep. 2019 Feb 6;19:100454. doi: 10.1016/j.ymgmr.2019.100454. eCollection 2019 Jun. Mol Genet Metab Rep. 2019. PMID: 30775256 Free PMC article. Review.
-
Clinical Characteristics, Renal Involvement, and Therapeutic Options of Pediatric Patients With Fabry Disease.Front Pediatr. 2022 Jun 1;10:908657. doi: 10.3389/fped.2022.908657. eCollection 2022. Front Pediatr. 2022. PMID: 35722479 Free PMC article. Review.
References
-
- Wanner C, Arad M, Baron R, Burlina A, Elliott PM, Feldt-Rasmussen U, et al. . European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab. 2018;124(3):189–203. - PubMed
-
- Germain DP, Altarescu G, Barriales-Villa R, Mignani R, Pawlaczyk K, Pieruzzi F, et al. . An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. 2022;137(1–2):49–61. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

