Review of cocaine-induced brain vascular and cellular function changes measured in vivo with optical imaging
- PMID: 40438148
- PMCID: PMC12118881
- DOI: 10.1117/1.NPh.12.S1.S14611
Review of cocaine-induced brain vascular and cellular function changes measured in vivo with optical imaging
Abstract
Significance: Cocaine exerts effects on vascular and cellular functions in the brain. The interactions among cerebrovasculature, neurons, and astrocytes and their dynamic changes during exposure complicate the understanding of its effects. Therefore, there is a need for simultaneous, multiparameter in vivo measurements to accurately distinguish these effects.
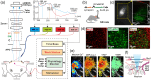
Aim: A multimodal optical imaging approach that is tailored to investigate cocaine's effects on cerebrovasculature, neurons, and astrocytes in high-spatiotemporal resolution and large field of view is presented with comparisons to other modalities.
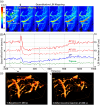
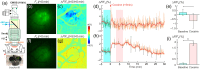
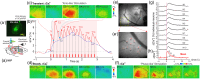
Approach: This approach integrates optical coherence tomography, fluorescence, and spectral absorption imaging to permit high-resolution imaging of 3D cerebrovessels, cerebral blood flow (CBF), changes in oxygenated/deoxygenated hemoglobin, and large-scale cellular activities via intracellular calcium fluorescence expressed through genetically encoded calcium indicators in the mouse cortex.
Results: Results show that cocaine induces vasoconstriction and reduces CBF, thus increasing the susceptibility of the brain to ischemia with chronic exposure. Moreover, cocaine alters neuronal activity and frontal responses to deep brain stimulation.
Conclusions: These findings on cocaine's effects on the neuro-astroglial-vascular network in the prefrontal cortex highlight the unique capacity of optical imaging to reveal the cellular and vascular mechanisms underlying cocaine's neurotoxic effects on brain function.
Keywords: addiction; cerebral blood flow; cocaine; hemoglobin oxygenation and deoxygenation; neuroimaging; optical imaging in vivo.
© 2025 The Authors.
Figures



Similar articles
-
Astrocytes mediate cerebral blood flow and neuronal response to cocaine in prefrontal cortex.Res Sq [Preprint]. 2023 Mar 20:rs.3.rs-2626090. doi: 10.21203/rs.3.rs-2626090/v1. Res Sq. 2023. Update in: Mol Psychiatry. 2024 Mar;29(3):820-834. doi: 10.1038/s41380-023-02373-9. PMID: 36993330 Free PMC article. Updated. Preprint.
-
Astrocytes modulate cerebral blood flow and neuronal response to cocaine in prefrontal cortex.Mol Psychiatry. 2024 Mar;29(3):820-834. doi: 10.1038/s41380-023-02373-9. Epub 2024 Jan 19. Mol Psychiatry. 2024. PMID: 38238549 Free PMC article.
-
Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo.Neuroimage. 2011 Jan 15;54(2):1130-9. doi: 10.1016/j.neuroimage.2010.08.045. Epub 2010 Sep 8. Neuroimage. 2011. PMID: 20804849 Free PMC article.
-
Optical detection of brain function: simultaneous imaging of cerebral vascular response, tissue metabolism, and cellular activity in vivo.Rev Neurosci. 2011;22(6):695-709. doi: 10.1515/RNS.2011.053. Epub 2011 Nov 18. Rev Neurosci. 2011. PMID: 22098474 Review.
-
In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review.
References
-
- He G. Q., et al. , “Cocaine-induced cerebrovasospasm and its possible mechanism of action,” J. Pharmacol. Exp. Ther. 268, 1532–1539 (1994).JPETAB - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

