Repetitive transcranial magnetic stimulation increases synaptic plasticity of cortical axons in the APP/PS1 amyloidosis mouse model
- PMID: 40438149
- PMCID: PMC12119023
- DOI: 10.1117/1.NPh.12.S1.S14613
Repetitive transcranial magnetic stimulation increases synaptic plasticity of cortical axons in the APP/PS1 amyloidosis mouse model
Abstract
Significance: Growing evidence highlights the therapeutic potential of repetitive transcranial magnetic stimulation (rTMS) in diseases causing dementias such as Alzheimer's disease (AD). However, individual responses to rTMS are variable, and its underlying neural mechanisms are not fully understood.
Aim: As synaptic dysfunction is one of the key mechanisms associated with cognitive deficits in dementia, we investigated the effect of rTMS on cortical synapses using an APP/PS1 amyloidosis mouse model of AD crossed with fluorescent reporters linked to the Thy-1 promoter.
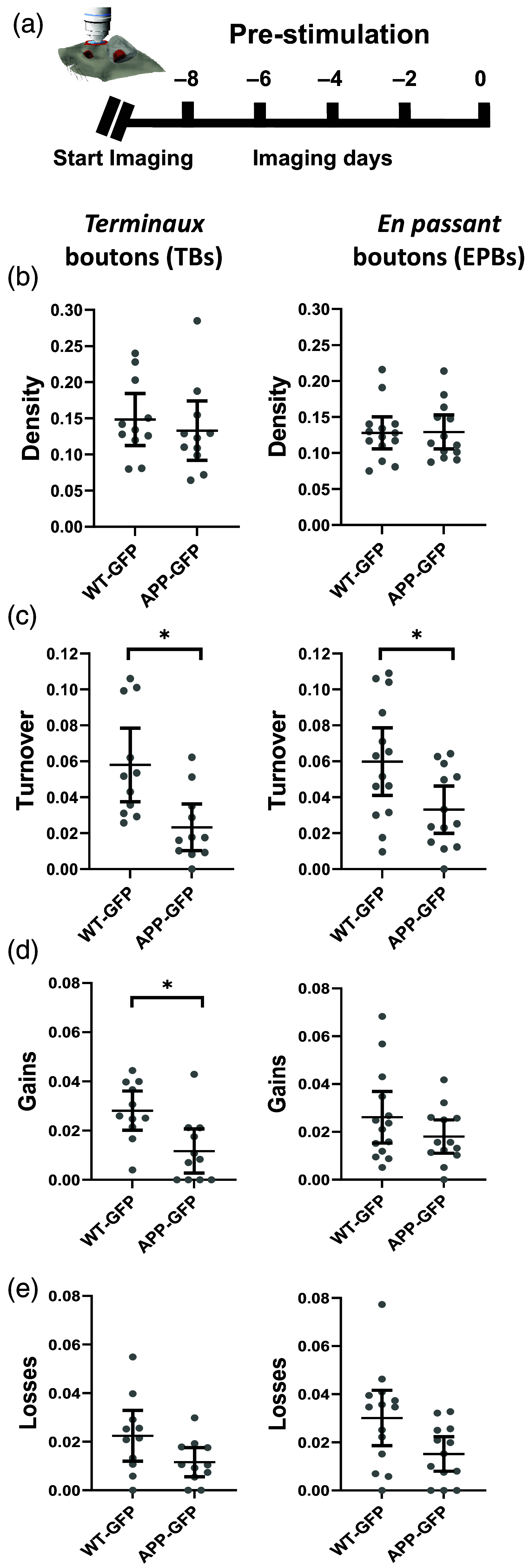
Approach: Using in vivo two-photon imaging, we characterized the plasticity of excitatory terminaux (TB) and en passant (EPB) axonal boutons at 48-h intervals for 8 days on either side of a single session of rTMS.
Results: We found both types of axonal boutons preserved the overall number of their synaptic outputs in wild type (WT) and APP/PS1 groups, pre- and post-stimulation. Both synapse types also showed a significantly reduced dynamic fraction in APP/PS1 compared with WT axons pre-stimulation. Following stimulation, the TB, but not EPB, dynamic fraction increased in both WT and APP/PS1 groups.
Conclusions: This suggests possible mechanisms of rTMS action that are cell type-specific and, together with previous findings of improved functional performance, present a potential clinical avenue for rTMS in the management of AD.
Keywords: axon; bouton; dementia; live imaging; synaptic plasticity; two-photon microscopy.
© 2025 The Authors.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

