Preferential survival of prebiotic metallopeptides in the presence of ultraviolet light
- PMID: 40438176
- PMCID: PMC12108965
- DOI: 10.1039/d5sc02170g
Preferential survival of prebiotic metallopeptides in the presence of ultraviolet light
Abstract
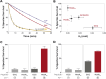
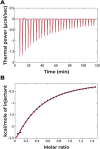
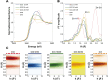
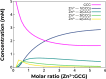
The transition from unregulated, prebiotic chemistry to metabolic-like systems capable of supporting an evolving protocell has remained difficult to explain. One hypothesis is that early catalysts began to prune the chemical landscape in a manner that facilitated the emergence of modern-day enzymes. As enzymes frequently rely on the intrinsic reactivity of metal ions, it follows that these early catalysts may have been metal ions coordinated to prebiotic peptides that have remained as core structures within extant proteins. Here, we demonstrate that UV light directly selects for the types of metal-binding peptide motifs found in biology. This is because bare cysteine is much more susceptible to photolysis than cysteine bound by a metal ion. Therefore, peptides with greater affinity for environmentally available metal ions, such as Fe2+ or Zn2+, are more stable. Our results are supported by mass spectrometry, calorimetry, X-ray absorption, NMR spectroscopy, transient absorption pump probe spectroscopy, and excited-state quantum-chemical calculations. Photostability arises from the ability of the metal ion to engage transiently generated reactive radical centers in a manner that prevents subsequent degradative processes. The data are consistent with the enrichment of a restricted set of high affinity, extant-like metallopeptides in surficial environments on the early Earth.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

