Deciphering tuberculosis: lysosome-centric insights into pathogenesis and therapies
- PMID: 40438237
- PMCID: PMC12116394
- DOI: 10.3389/fcimb.2025.1582037
Deciphering tuberculosis: lysosome-centric insights into pathogenesis and therapies
Abstract
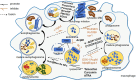
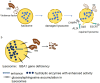
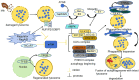
Tuberculosis is a widely spread disease caused by Mycobacterium tuberculosis (Mtb). The pathogenicity of the pathogen is closely associated with the immune defense mechanisms of the host cells. As key cellular degradation and metabolic centers, lysosomes critically regulate tuberculosis infection. When Mtb invades the host, it is taken up by macrophages and enters phagosomes. Subsequently, the phagosomes fuse with lysosomes and form phagolysosomes, which eliminate the pathogenic bacteria through the acidic environment and hydrolytic enzymes within lysosomes. However, Mtb can interfere with the normal functions of lysosomes through various strategies. It can secrete specific factors (such as ESAT-6, ppk-1, and AcpM) to inhibit the acidification of lysosomes, enzyme activity, and the fusion of phagosomes and lysosomes, thereby enabling Mtb proliferation within host cells. An in-depth exploration of the mechanism of the interaction between Mtb and lysosomes will both uncover bacterial immune evasion strategies and identify novel anti-tuberculosis therapeutic targets.
Keywords: Mycobacterium tuberculosis (Mtb); interaction; lysosomes; mechanism; treatment.
Copyright © 2025 Bao, Zhang, Feng, Hong, Gao and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation.PLoS Pathog. 2018 Apr 30;14(4):e1007011. doi: 10.1371/journal.ppat.1007011. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29709019 Free PMC article.
-
Mycobacterium tuberculosis VII secretion system effector molecule Rv2347c blocks the maturation of phagosomes and activates the STING/TBK1 signaling pathway to inhibit cell autophagy.Microbiol Spectr. 2024 Nov 5;12(11):e0118824. doi: 10.1128/spectrum.01188-24. Epub 2024 Sep 23. Microbiol Spectr. 2024. PMID: 39313213 Free PMC article.
-
Macrophage defense mechanisms against intracellular bacteria.Immunol Rev. 2015 Mar;264(1):182-203. doi: 10.1111/imr.12266. Immunol Rev. 2015. PMID: 25703560 Free PMC article. Review.
-
A Rab20-Dependent Membrane Trafficking Pathway Controls M. tuberculosis Replication by Regulating Phagosome Spaciousness and Integrity.Cell Host Microbe. 2017 May 10;21(5):619-628.e5. doi: 10.1016/j.chom.2017.04.004. Cell Host Microbe. 2017. PMID: 28494243 Free PMC article.
-
Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv.FEBS J. 2010 Jun;277(11):2416-27. doi: 10.1111/j.1742-4658.2010.07666.x. FEBS J. 2010. PMID: 20553485 Review.
References
-
- Ahmad F., Fatima N., Ahmad S., Upadhyay T. K., Jain P., Saeed M., et al. . (2024). Treatment of Mycobacterium tuberculosis infected macrophages with rifabutin loaded β-glucan microparticles induces macroautophagy mediated bacillary killing. Int. J. Biol. Macromol 283, 137256. doi: 10.1016/j.ijbiomac.2024.137256 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

