Disrupted functional connectome in a rodent model of autism during social isolation
- PMID: 40438462
- PMCID: PMC12116437
- DOI: 10.3389/fncir.2025.1525130
Disrupted functional connectome in a rodent model of autism during social isolation
Abstract
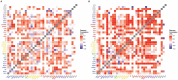
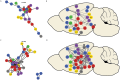
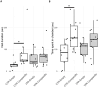
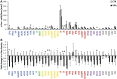
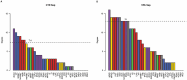
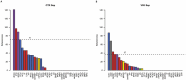
Autism spectrum disorder (ASD) is associated with disruptions in social behavior and the neural circuitry behind it. Very little data is available on the mechanisms that are responsible for the lack of motivation to reunite with conspecifics during isolation. It is as important to investigate the neural changes that reduce motivation to end social isolation, as those underlying the reactions to social stimuli. Using a rodent model of prenatal valproic acid (VPA) exposure, we investigated how social isolation affects the neural activation of key brain nuclei involved in social processing and stress regulation. Juvenile male C57BL/6 mice were treated prenatally with VPA or saline (CTR) and subjected to 24 h of social isolation from their cage mates, with neural activity assessed via c-Fos immunohistochemistry. Based on correlational activations we reconstructed and analyzed the functional connectome of the observed brain regions. Control animals exhibited elevated c-Fos expression in the regions central to the mesolimbic reward system (MRS), social brain network (SBN), and stress-related networks, with the interpeduncular nucleus (IPN) at the core, compared to VPA-treated animals. Functional network analysis revealed a more widespread but less specific pattern of connectivity in VPA-treated animals. These findings suggest that prenatal VPA exposure disrupts certain neural circuits related to social behavior and stress regulation, offering an insight into the altered perception of social isolation in ASD models, and highlighting potential therapeutic targets.
Keywords: VPA; autism spectrum disorder; functional connectivity; social decision making network; social isolation; stress regulating network; valproic acid.
Copyright © 2025 Kemecsei, Dániel-Papp, Balazs, Ghebrihiwet Tewelde, Csillag and Zachar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
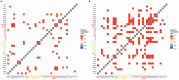
Similar articles
-
Prenatal valproic acid exposure disrupts tonotopic c-Fos expression in the rat brainstem.Neuroscience. 2016 Jun 2;324:511-23. doi: 10.1016/j.neuroscience.2016.01.030. Epub 2016 Jan 25. Neuroscience. 2016. PMID: 27094734
-
Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder.Sci Rep. 2021 Dec 6;11(1):23471. doi: 10.1038/s41598-021-02994-6. Sci Rep. 2021. PMID: 34873263 Free PMC article.
-
Prenatal valproic acid exposure disrupts tonotopic c-Fos expression in the rat brainstem.Neuroscience. 2015 Dec 17;311:349-61. doi: 10.1016/j.neuroscience.2015.10.043. Epub 2015 Oct 28. Neuroscience. 2015. Corrected and republished in: Neuroscience. 2016 Jun 2;324:511-23. doi: 10.1016/j.neuroscience.2016.01.030. PMID: 26518464 Corrected and republished.
-
Altered attentional processing in male and female rats in a prenatal valproic acid exposure model of autism spectrum disorder.Autism Res. 2017 Dec;10(12):1929-1944. doi: 10.1002/aur.1852. Epub 2017 Aug 29. Autism Res. 2017. PMID: 28851114
-
Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder.Neuropharmacology. 2019 Nov 15;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. Epub 2019 Jan 9. Neuropharmacology. 2019. PMID: 30639388 Review.
References
-
- Ádám Á., Kemecsei R., Company V., Murcia-Ramón R., Juarez I., Gerecsei L. I., et al. . (2020). Gestational exposure to sodium valproate disrupts fasciculation of the Mesotelencephalic dopaminergic tract, with a selective reduction of dopaminergic output from the ventral tegmental area. Front. Neuroanat. 14:29. doi: 10.3389/fnana.2020.00029, PMID: - DOI - PMC - PubMed
-
- Atlas Thumbnails (2024) Allen brain atlas: Mouse brain. Available online at: https://mouse.brain-map.org/experiment/thumbnails/100048576?image_type=a... (accessed August 9, 2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

