Advances in photocatalytic research on decarboxylative trifluoromethylation of trifluoroacetic acid and derivatives
- PMID: 40438535
- PMCID: PMC12116483
- DOI: 10.3389/fchem.2025.1602003
Advances in photocatalytic research on decarboxylative trifluoromethylation of trifluoroacetic acid and derivatives
Abstract
Trifluoromethylation stands as a pivotal technology in modern synthetic chemistry, playing an indispensable role in drug design, functional material development, and agrochemical innovation. With the growing emphasis on green chemistry principles, the pursuit of environmentally benign trifluoromethylation strategies has emerged as a critical research frontier. Trifluoroacetic acid (TFA), characterized by its cost-effectiveness, stability, and low toxicity, has become a promising alternative to conventional trifluoromethylation reagents. This review systematically summarizes advancements in photocatalytic decarboxylative trifluoromethylation using TFA and its derivatives over the past decade, focusing on three key activation mechanisms: single-electron transfer (SET), electron donor-acceptor (EDA) complex-mediated pathways, and ligand-to-metal charge transfer (LMCT). This paradigm shift is driven by the intrinsic limitations of conventional thermal decarboxylation, particularly its reliance on harsh conditions and significant environmental burdens. In contrast, photocatalytic strategies enable efficient C-CF3 bond construction under mild conditions, offering a modular platform for synthesizing fluorinated functional molecules. Strategic research priorities should focus on overcoming fundamental challenges, including but not limited to optimizing photosensitizer catalytic efficiency, establishing regioselective manipulation strategies, and engineering multicomponent tandem reaction systems to achieve trifluoromethylation methodologies under mild conditions. Furthermore, the integration of mechanistic investigations with artificial intelligence-driven reaction prediction will accelerate the advancement of precision trifluoromethylation technologies. This progress is anticipated to provide sustainable synthetic solutions for next-generation fluorinated pharmaceuticals and advanced functional materials, effectively bridging the innovation gap between academic research and industrial implementation.
Keywords: decarboxylation; photocatalysis; radical; trifluoroacetic acid (TFA); trifluoromethylation.
Copyright © 2025 Tan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


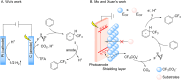
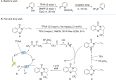




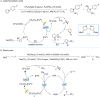
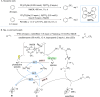
Similar articles
-
Photocatalytic fluoroalkylation by ligand-to-metal charge transfer.Front Chem. 2024 Sep 6;12:1481342. doi: 10.3389/fchem.2024.1481342. eCollection 2024. Front Chem. 2024. PMID: 39308850 Free PMC article. Review.
-
Electrophotochemical Synthesis Facilitated Trifluoromethylation of Arenes Using Trifluoroacetic Acid.J Am Chem Soc. 2023 Nov 3. doi: 10.1021/jacs.3c10148. Online ahead of print. J Am Chem Soc. 2023. PMID: 37920956
-
Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero)arenes with trifluoroacetic acid.Nat Commun. 2017 Feb 6;8:14353. doi: 10.1038/ncomms14353. Nat Commun. 2017. PMID: 28165474 Free PMC article.
-
Recent Progress of Chemical Reactions Induced by Contact Electrification.Molecules. 2025 Jan 27;30(3):584. doi: 10.3390/molecules30030584. Molecules. 2025. PMID: 39942688 Free PMC article. Review.
-
Direct C-H trifluoromethylation of di- and trisubstituted alkenes by photoredox catalysis.Beilstein J Org Chem. 2014 May 12;10:1099-106. doi: 10.3762/bjoc.10.108. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24991259 Free PMC article.
References
-
- Andreev V. N., Grinberg V. A., Dedov A. G., Loktev A. S., Mayorova N. A., Moiseev I. I., et al. (2013). Anodic trifluoromethylation of 10-undecylenic acid. Russ. J. Electrochem. 49, 996–1000. 10.1134/s1023193513100030 - DOI
-
- Barton D. H. R., Lacher B., Zard S. Z. (1986). The invention of new radical chain-reactions. Part 13: generation and reactivity of perfluoroalkyl radicals from thiohydroxamic esters. Tetrahedron 42, 2325–2328. 10.1016/s0040-4020(01)90613-1 - DOI
-
- Bazyar Z., Hosseini-Sarvari M. (2019). Au@ZnO core-shell: scalable photocatalytic trifluoromethylation using CF3CO2Na as an inexpensive reagent under visible light irradiation. Org. Process Res. Dev. 23, 2345–2353. 10.1021/acs.oprd.9b00225 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

