Same-Day Magnetic Resonance-Guided Single-Fraction Stereotactic Body Radiation Therapy for Painful Non-Spine Bone Metastases - A Single-Center Study ("BONE SHOT")
- PMID: 40438564
- PMCID: PMC12117186
- DOI: 10.1016/j.ctro.2025.100966
Same-Day Magnetic Resonance-Guided Single-Fraction Stereotactic Body Radiation Therapy for Painful Non-Spine Bone Metastases - A Single-Center Study ("BONE SHOT")
Abstract
Introduction and background: There is evidence for efficacy of high-dose single-fraction stereotactic body radiotherapy (SF-SBRT) for painful non-spine bone metastases (NSBMs). This study ("BONE SHOT") assessed feasibility of same-day magnetic resonance-guided (MRg) planning and SF-SBRT delivery, recorded toxicity and assessed efficacy for treating metastatic patients with NSBMs.
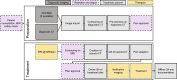
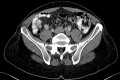
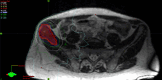
Materials and methods: Patients with painful (≥3/10 points on a 0-10 numeric rating scale (NRS) for pain) and radiologically confirmed NSBMs from solid organ malignancies were eligible for this prospectively acquired, single-center study. Patients received MRg-SF-SBRT via MR-Linac (ViewRay®) with same-day consultation, consent, planning and treatment. Drop-out rate, procedure times, acute toxicity and pain response were recorded.
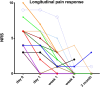
Results: Between June 2019 and June 2020, 13 patients with 15 NSBMs were treated per protocol. Mean patient age was 64 (range, 30-87) years; most common primary cancer was gastrointestinal malignancies (38.5 %); most commonly treated site was pelvis (53.3 %). All workflows were completed as planned. Median on-table time for contouring, planning and delivery was 65 (range, 57-112) minutes. Treatments were well tolerated; one patient developed "pain flair"; no grade ≥ 3 toxicities were registered. At one week following SBRT, overall and complete pain response rates were 73.3 % and 20.0 %, respectively, which evolved to 66.7 % and 53.3 % at four weeks after SBRT; median pre-treatment pain score was 6 points, which was reduced by a median of 5 points (P = 0.0028) at four weeks.
Conclusion: The same-day MRg-SF-SBRT workflow for NSBMs was feasible, safe, and preliminary results indicate promising efficacy, warranting future trials investigating this intervention.
Keywords: Bone metastases; MR-Linac; Palliative radiotherapy; SBRT.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Ryan C., Stoltzfus KC., Horn S., Chen H., Louie AV., Lehrer EJ., et al. Epidemiology of bone metastases. Bone. 2020 - PubMed
-
- Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. 2012;24(2):112–124. - PubMed
-
- Imano N., Saito T., Hoskin P., Nakamura N., Ito K., Yorozu A., et al. Pain response rates after conventional radiation therapy for bone metastases assessed using international consensus pain response endpoints: a systematic review and meta-analysis of initial radiation therapy and reirradiation. Int J Radiat Oncol Biol Phys. 2023;116(4):739–746. - PubMed
LinkOut - more resources
Full Text Sources

