Structure, morphology, optical properties, and Judd-Ofelt analysis of YP(1- x)V x O4:Eu3+ materials synthesized by the combustion method
- PMID: 40438666
- PMCID: PMC12107622
- DOI: 10.1039/d4na01052c
Structure, morphology, optical properties, and Judd-Ofelt analysis of YP(1- x)V x O4:Eu3+ materials synthesized by the combustion method
Abstract
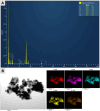
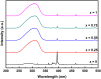
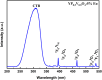
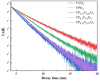
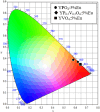
YP(1-x)V x O4:Eu3+ materials were synthesized via a simple combustion method. Material characterization illustrated the formation of spherical particles with a tetragonal crystal structure and a uniform size of 20 nm, although aggregation was observed. Fluorescence spectroscopy was then employed to explore the optical characteristics, revealing key insights into the luminescent behavior of the as-prepared materials. A detailed examination of the branching ratio of the 5D0 → 7F2 electronic transition relative to the 5D0 → 7F1 transition was performed, which is closely tied to the symmetry of the local environment of the Eu3+ activators. This investigation utilized Judd-Ofelt theory to calculate intensity and emission parameters. Additionally, the fluorescence lifetime of the material was measured under various V/P ratios, elucidating the relationship between these variables. Finally, the emission color and correlated color temperature (CCT) of the synthesized material were evaluated through the CIE 1931 chromaticity diagram, confirming its potential for use in optical applications based on its tunable emission characteristics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khan L. U. and Khan Z. U., in Handbook of Materials Characterization, Springer International Publishing, Cham, 2018, pp. 345–404
-
- Bünzli J.-C. G. Eliseeva S. V. J. Rare Earths. 2010;28:824–842. doi: 10.1016/S1002-0721(09)60208-8. - DOI
LinkOut - more resources
Full Text Sources

