Efficacy of Total-Body Irradiation-based Intensified Myeloablative Regimens for Acute Leukemia-An International Collaborative Study
- PMID: 40438703
- PMCID: PMC12118588
- DOI: 10.1002/jha2.70061
Efficacy of Total-Body Irradiation-based Intensified Myeloablative Regimens for Acute Leukemia-An International Collaborative Study
Abstract
Background: In this study, we compared outcomes of intensified myeloablative conditioning regimens using large registry data from Japan (Japanese Society for Transplantation and Cellular Therapy) and the United States (Center for International Blood and Marrow Transplant Research).
Methods: Adult patients who underwent their first myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) for acute leukemia in remission between 2010 and 2018 using conditioning regimens of cyclophosphamide plus total-body irradiation (CY/TBI), CY/TBI+cytarabine (AraC), or CY/TBI+etoposide (VP16) were included.
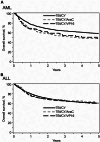
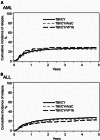
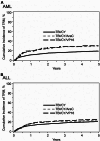
Results: The acute myeloid leukemia (AML) cohort (N = 480, 38.8%) indicated that overall survival (OS) was poorer in CY/TBI+AraC (hazard ratio [HR] 1.46, p < 0.001) and CY/TBI+VP16 (HR 1.39, p = 0.059) compared to CY/TBI. Relapse was not suppressed, while treatment-related mortality (TRM) was significantly higher (HR 1.78 and 1.74, p < 0.001 and 0.018, respectively). In the acute lymphoblastic leukemia (ALL) cohort (N = 3901, 61.2%), OS was comparable among these regimens. With intensified regimens, relapse was significantly suppressed in CY/TBI+VP16 (HR 0.74, p = 0.005), while TRM was higher (HR 1.21, p = 0.077). No interactions were observed regarding the country.
Conclusion: In AML adding AraC and VP16 to CY/TBI had an adverse effect on OS. Conversely, in ALL, adding VP16 or AraC to CY/TBI did not affect survival, but the addition of VP16 reduced the risk of relapse.
Clinical trial registration: The authors have confirmed clinical trial registration is not needed for this submission.
Keywords: acute leukemia; international collaborative study; myeloablative conditioning.
© 2025 The Author(s). eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Chhabra reports institutional research funding: Janssen, C4 Therapeutics, AbbVie, CARSgen, Takeda; honoraria for the advisory board: GSK, Sanofi, Janssen. Dr. Battiwalla reports compensation for research support from Novartis. Dr. Steinberg reports speaking for Jazz Pharmaceuticals Dr. Frangoul reports consulting for Vertex Pharmaceuticals and Editas Medicine. Dr. Lazarus reports consulting for Actinium Pharmaceuticals, Inc., Partner Therapeutics, Jazz Pharmaceuticals, Pluristem Therapeutics Inc., CSL Behring, Seattle Genetics; and promotional speaker for AstraZeneca; data safety monitoring for Bristol‐Myers Squibb and BioSight. Dr. Yared reports honoraria from participating in advisory board meetings with Incyte, Sanofi, Kadmon, and Omeros. Dr. Sharma reports consulting for Spotlight Therapeutics, Medexus Inc., Vertex Pharmaceuticals, and Sangamo Therapeutics; research funding from CRISPR Therapeutics. Clinical Trial site‐PI: CRISPR Therapeutics, Vertex Pharmaceuticals, Novartis Pharmaceuticals, Magenta Therapeutics, Beam Therapeutics, Honoraria: Vindico Medical Education. Dr. Dias reports the following disclaimer: This article was prepared by Dr Ajoy Dias in his personal capacity. The opinions expressed in this manuscript are the author's own and do not reflect the view of the National Institute of Health, the Department of Health and Human Services, or the United States government. Dr. Zeidan reports participation in advisory boards, consulted and/or received honoraria from AbbVie, Agios, Pfizer, Astellas, Astex, ALX Oncology, Amgen, Akeso pharma, BeiGene, BMS/Celgene, Boehringer‐Ingelheim, BioCryst, Chiesi, Daiichi Sankyo, Epizyme, Faron, Geron, Gilead, Genentech, Glycomimetics, Hikma, Ionis, Janssen, Kura, Keros, Karyopharm, Kyowa Kirin, Lava Therapeutics, Mendus, Notable, Novartis, Otsuka, Orum, Taiho, Takeda, Treadwell, Syndax, Sumitomo, STCube, Schrodinger, Servier, Syros, Vincerx, Regeneron, Rigel, and Zentalis. Dr. Rangarajan reports serving as the BMT Medical Monitor for NMDP (no financial reimbursements) and having served as an honorary consultant for Medexus. Dr. Hashmi reports compensation from advisory boards in Asia for GVHD and BMT drugs and reports giving talks on educational CME programs that are sponsored by pharma. Dr. Atsuta reports consulting for JCR Pharmaceuticals Co., Ltd, Kyowa Kirin Co., Ltd; honoraria from Meiji Seika Pharma Co, Ltd.; and lecturing for Otsuka Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Novartis Pharma KK, and AbbVie GK. Dr. Kako reports honoraria from Nippon Shinyaku Co., Ltd. Dr. Kanda reports grants from Kyowa Kirin, Sumitomo Pharma, Chugai, Takeda, and Eisai, and personal fees from Pfizer, Sumitomo Pharma, Novartis, Sanofi, Chugai, Symbio, Bristol Myers Squip, And Janssen, outside the submitted work. Dr. Teshima reports personal fees from Merck Sharp & Dohme; grants and personal fees from Kyowa Kirin; personal fees from Takeda, Pfizer, and Bristol‐Myers Squibb; grants from Chugai, Sanofi, Astellas, Teijin Pharma, Fuji Pharma, Nippon Shinyaku; non‐financial support from Janssen; and grants, personal fees, and non‐financial support from Novartis, outside the submitted work.
Figures
References
-
- Arai Y., Kondo T., Shigematsu A., et al., “High‐dose Cytarabine Added to CY/TBI Improves the Prognosis of Cord Blood Transplantation for Acute Lymphoblastic Leukemia in Adults: A Retrospective Cohort Study,” Bone Marrow Transplantation 51, no. 12 (2016): 1636–1639. - PubMed
-
- Inamoto Y., Nishida T., Suzuki R., et al., “Significance of Additional High‐dose Cytarabine in Combination With Cyclophosphamide plus Total Body Irradiation Regimen for Allogeneic Stem Cell Transplantation,” Bone Marrow Transplantation 39, no. 1 (2007): 25–30. - PubMed
-
- Arai Y., Kondo T., Shigematsu A., et al., “Improved Prognosis With Additional Medium‐dose VP16 to CY/TBI in Allogeneic Transplantation for High Risk ALL in Adults,” American Journal of Hematology 93, no. 1 (2018): 47–57. - PubMed
