Reirradiation: Standards, challenges, and patient-focused strategies across tumor types
- PMID: 40438993
- PMCID: PMC12593330
- DOI: 10.3322/caac.70016
Reirradiation: Standards, challenges, and patient-focused strategies across tumor types
Abstract
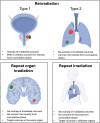
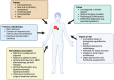
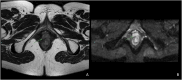
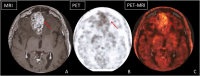
Reirradiation (reRT), defined as administering a course of radiation therapy to a specific area previously irradiated, is an evolving treatment strategy for locoregionally recurrent cancer that offers significant potential and poses inherent challenges. Advances in such techniques as intensity-modulated and stereotactic body radiation therapy have improved precision, making reRT a viable option for complex scenarios previously deemed high-risk. Nevertheless, reRT remains associated with substantial risks-including life-threatening side effects, functional impairments, and psychosocial effects-which must be carefully balanced against the patient's overall health and the likelihood of achieving cancer control or palliation. Patient selection is essential to optimize outcomes while mitigating risks. Decisions should account for tumor characteristics at the time of primary diagnosis and recurrence, elapsed time since prior treatment, the possibility of delivering meaningful doses to the tumor, and the cumulative irradiation tolerance of normal tissues. Advanced imaging modalities, such as functional magnetic resonance imaging and fluorine-18-labeled fluorodeoxyglucose-positron emission tomography, are important for distinguishing recurrences from treatment-induced changes, refining treatment targets, and minimizing exposure to healthy tissue. Combined treatment with systemic regimens-targeted therapies and immunotherapy in particular-offers promising opportunities but requires coordination to manage side effects. Standardized guidelines, such as those from the European Society of Therapeutic Radiology and Oncology-European Society for Research and Treatment of Cancer, are essential for improving the consistency of reporting, guiding clinical decision making, and fostering patient-centered care. Multidisciplinary collaboration and ongoing research, particularly through clinical trials, are central to fully exploiting reRT strategies. In addition, the development of innovative techniques, such as proton therapy, would likely enable safer treatments. These efforts aim to improve the therapeutic balance of reRT, enhancing outcomes and quality of life.
Keywords: advanced imaging techniques; decision‐making; locoregional recurrence; radiation oncology; reirradiation.
© 2025 The Author(s). CA: A Cancer Journal for Clinicians published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Dr Kevin Chua reports support for professional activities from AstraZeneca, F. Hoffmann‐La Roche, Suzhou Liangyihui Network Technology, and Varian Medical Systems; and travel support from AstraZeneca outside the submitted work. Prof. Dorota Gabrys reports support for professional activities from Seimens Healthineers International AG outside the submitted work. Prof. Maximillian Niyazi reports grants/contracts from Brainlab Inc. and support for other professional activities from AstraZeneca AB outside the submitted work. Dr Kelly Paradis reports support for professional activities from Varian Medical Systems outside the submitted work. Dr Nicole C. Schmitt reports grants/contracts from Astex Pharmaceuticals; and personal/consulting fees from Checkpoint Surgical Inc., GeoVax, Johnson & Johnson, Regeneron, Sensorion, and Synergy Research Inc. outside the submitted work. Prof. Sue S. Yom reports grants/contracts from Bristol Myers Squibb Company, Johnson & Johnson Health Care Systems Inc., Merck, and Nanobiotix outside the submitted work. Prof. Nicholaus Andratschke reports grants/contracts from ViewRay Inc. outside the submitted work. The remaining authors disclosed no conflicts of interest.
Figures



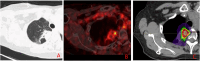
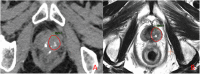
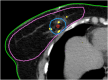
References
-
- Andratschke N, Willmann J, Appelt AL, et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus on re‐irradiation: definition, reporting, and clinical decision making. Lancet Oncol. 2022;23(10):e469‐e478. doi: 10.1016/s1470-2045(22)00447-8 - DOI - PubMed
-
- Dijkstra EA, Nilsson PJ, Hospers GAP, et al. Locoregional failure during and after short‐course radiotherapy followed by chemotherapy and surgery compared with long‐course chemoradiotherapy and surgery: a 5‐year follow‐up of the RAPIDO trial. Ann Surg. 2023;278(4):e766‐e772. doi: 10.1097/sla.0000000000005799 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

