Serum protein biomarker signature of Duchenne muscular dystrophy
- PMID: 40438995
- PMCID: PMC12265423
- DOI: 10.4081/ejtm.2025.13956
Serum protein biomarker signature of Duchenne muscular dystrophy
Abstract
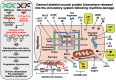
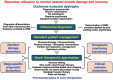
In contrast to invasive skeletal muscle biopsies and the associated complexity of tissue sampling techniques and potential detrimental side effects, the alternative application of liquid biopsy procedures has considerable advantages concerning minimal invasiveness, repeated sampling options, assay robustness and cost effectiveness. This article outlines the current status of serum biomarkers used for diagnosing and characterizing Duchenne muscular dystrophy (DMD), a primary muscle wasting disease of early childhood due to primary abnormalities in the extremely large DMD gene. Reviewed are important aspects of the discovery, characterization and diagnostic value of biofluid-based protein markers of dystrophinopathy. This includes an overview of traditional general skeletal muscle damage markers, such as creatine kinase, myoglobin and lactate dehydrogenase, which have been used for many decades in clinical applications to evaluate patients with muscular weakness. In addition, this article outlines the biochemical identification of novel biomarker candidates focusing on the usage of mass spectrometry-based proteomic surveys to establish comprehensive profiles of protein alterations in dystrophinopathy. Pathoproteomic serum markers of myonecrosis with great potential for improved patient screening, differential diagnosis, stage-specific prognosis and therapeutic monitoring include specific isoforms of muscle-derived cytosolic proteins, such as carbonic anhydrase isoform CA3 and fatty acid binding protein FABP3, as well as sarcomeric proteins, including specific isoforms of myosin light chain, myosin binding protein, troponin, and myomesin, in addition to peptide fragments derived from the giant protein titin. Biofluid-associated marker proteins of reactive myofibrosis include the extracellular matrix proteins fibronectin, osteopontin, collagen and matrix-metalloproteinases.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gambetta KE, McCulloch MA, Lal AK, et al. Diversity of dystrophin gene mutations and disease progression in a contemporary cohort of Duchenne muscular dystrophy. Pediatr Cardiol 2022;43:855-67. - PubMed
-
- Ohlendieck K, Matsumura K, Ionasescu V V, et al. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology 1993;43:795-800. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

