Efficacy of RTS,S/AS01E Only Seen in Baseline Parasitemic and Not Baseline Aparasitemic Plasmodium falciparum-Exposed, Drug-Treated Kenyan Adults
- PMID: 40439411
- PMCID: PMC12455321
- DOI: 10.1093/infdis/jiaf274
Efficacy of RTS,S/AS01E Only Seen in Baseline Parasitemic and Not Baseline Aparasitemic Plasmodium falciparum-Exposed, Drug-Treated Kenyan Adults
Abstract
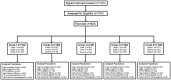
Background: RTS,S/AS01 vaccine efficacy (VE) was previously shown as lower in African adults than in malaria-naive US adults, potentially due to concurrent Plasmodium falciparum infections. We investigated whether treatment of infection prior to vaccination would lead to improved VE and immunogenicity.
Methods: A phase 2b study in Kenyan adults evaluated the efficacy of RTS,S/AS01E in conjunction with antimalarial chemopreventive drugs. Participants, grouped by baseline presence or absence of P. falciparum infections, were randomized to receive RTS,S/AS01E or rabies vaccine. Four groups received antimalarial drugs prior to immunization and were followed for 6 months to assess P. falciparum infection. We included an additional group not treated with antimalarial drugs for immunological assessment.
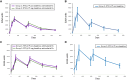
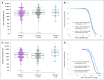
Results: VE (RTS,S/AS01E vs rabies vaccine) was 34.8% (95% confidence interval [CI], 8.9% to 53.4%) and -24.0% (95% CI, -97% to 22.4%) in baseline P. falciparum-positive and P. falciparum-negative participants, respectively. In RTS,S/AS01E recipients, there were no statistical differences in anticircumsporozoite (anti-CS) antibody titers in baseline P. falciparum-positive or P. falciparum-negative participants, or in susceptibility to infection during the postvaccination follow-up period. Drug treatment did not improve anti-CS antibody titers.
Conclusions: Treating P. falciparum infections during vaccination does not result in increased VE. Anti-CS antibody responses to vaccination do not differ with baseline P. falciparum infection status, drug treatment, or susceptibility to P. falciparum infections.
Clinical trials registration: NCT04661579; PACTR202006896481432.
Keywords: CSP antibody titers and infection; RTS,S/AS01; antimalarial drug treatment and malaria vaccine; malaria vaccine in African adults; vaccine-induced antibodies and infection.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. L. is an employee of the GSK group of companies and has restricted shares in the GSK group of companies. All other authors report no potential conflicts except for those listed under the Financial Support section. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- World Health Organization . Mosquirix prequalification, 2022. https://extranet.who.int/prequal/vaccines/p/mosquirix. Accessed 15 July 2022.
-
- World Health Organization . Malaria vaccine: WHO position paper—May 2024, 2024. https://www.who.int/publications/i/item/who-wer-9919-225-248. Accessed 1 May 2024.
-
- Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis 2016; 214:762–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

