Impact of In-Hospital PCSK9 Inhibition on Myocardial Inflammation After Myocardial Infarction: A Randomized Clinical Trial
- PMID: 40439630
- PMCID: PMC12230458
- DOI: 10.1016/j.jacbts.2025.03.010
Impact of In-Hospital PCSK9 Inhibition on Myocardial Inflammation After Myocardial Infarction: A Randomized Clinical Trial
Abstract
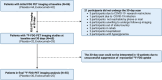
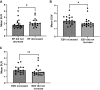
In a randomized trial with 55 participants from the EVACS (Evolocumab in Acute Coronary Syndrome) trials, patients with non-ST-segment elevation myocardial infarction (MI) or ST-segment elevation MI received a single dose of evolocumab or placebo, with myocardial inflammation assessed via 18F-fluorodeoxyglucose positron emission tomography scans at baseline and at 30 days. Evolocumab significantly reduced inflammation (SUVmean) compared with placebo. PCSK9 levels at 30 days correlated with SUVmean, and higher SUVmean was linked to increased end-systolic volume at 6 months. These findings suggest that early PCSK9 inhibition reduces post-MI myocardial inflammation and may influence cardiac remodeling in the months following the acute event.
Keywords: (18)F-FDG-PET imaging; PCSK9 inhibition; acute myocardial infarction; myocardial inflammation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by Amgen Inc. Amgen, the funder of the study, had no role in the design of the study, the collection, management, or interpretation of the data, or the statistical analysis. The funder reviewed the first submitted version of the paper but was not involved in the writing or approval of the paper or the decision to submit the paper for publication. Dr Blaha has received grants from Amgen, Bayer, Novo Nordisk, and the U.S. Food and Drug Administration; and has served on advisory boards for Novartis, Novo Nordisk, Bayer, Roche, Merck, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Agepha, New Amsterdam, Genentech. Dr Hays is supported by National Institutes of Health grants R35HL1431598 and R01HL147660. Dr Leucker has received research grants from the American Heart Association Career Development Award: 19CDA34760040, Merck, Novartis and Amgen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Biasucci L.M., Liuzzo G., Grillo R.L., et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855–860. - PubMed
-
- Mueller C., Buettner H.J., Hodgson J.M., et al. Inflammation and long-term mortality after non-ST elevation acute coronary syndrome treated with a very early invasive strategy in 1042 consecutive patients. Circulation. 2002;105:1412–1415. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

